Amino Alcohols as Ligands for Nickel-Catalyzed Suzuki Reactions of Unactivated Alkyl Halides, Including Secondary Alkyl Chlorides, with Arylboronic Acids
Francisco González-Bobes and Gregory C. Fu*
*Department of Chemistry, Massachusetts Institute of Technology, Cambridge,
Massachusetts 02139, Email: gcf mit.edu
mit.edu
F. González-Bobes, G. C. Fu, J. Am. Chem. Soc., 2006, 128, 5360-5361.
DOI: 10.1021/ja0613761


Abstract
Suzuki cross-coupling reactions of an unprecedented array of unactivated primary and secondary alkyl halides with arylboronic acids can be accomplished through the use of nickel/amino alcohol-based catalysts. Both the nickel precatalysts and the amino alcohols are commcercially available and air-stable.
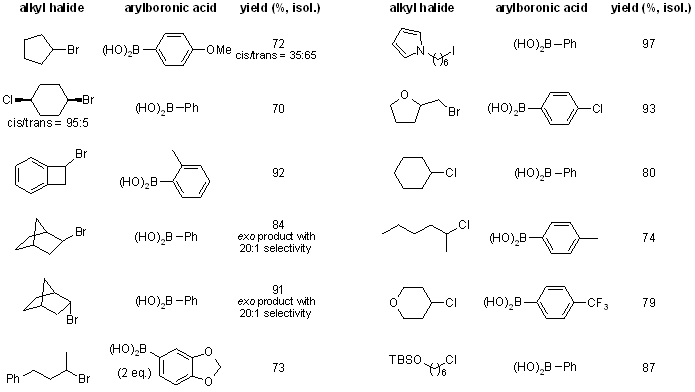
see article for more examples
Alkyl-Alkyl Suzuki Cross-Couplings of Unactivated Secondary Alkyl Halides at Room Temperature
B. Saito, G. C. Fu, J. Am. Chem. Soc., 2007, 129, 9602-9603.
J. H. Kirchhoff, M. R. Netherton, I. D. Hill, G. C. Fu, J. Am. Chem. Soc., 2002, 124, 13662-13663.
Room-Temperature Hiyama Cross-Couplings of Arylsilanes with Alkyl Bromides and Iodides
J.-Y. Lee, G. C. Fu, J. Am. Chem. Soc., 2003, 125, 5616-5617.
Key Words
Suzuki Coupling, Substituted Arenes
ID: J48-Y2006-1980
