Mild and Efficient Pentafluorophenylammonium Triflate (PFPAT)-Catalyzed C-Acylations of Enol Silyl Ethers or Ketene Silyl (Thio)Acetals with Acid Chlorides
Akira Iida, Jun Osada, Ryohei Nagase, Tomonori Misaki and Yoo Tanabe*
*Department of Chemistry, School of Science and Technology, Kwansei Gakuin
University, 2-1 Gakuen, Sanda, Hyogo 669-1337, Japan, Email: tanabe kwansei.ac.jp
kwansei.ac.jp
A. Iida, J. Osada, R. Nagase, T. Misaki, Y. Tanabe, Org. Lett., 2007, 9, 1859-1862.
DOI: 10.1021/ol070191b

Abstract
Pentafluorophenylammonium triflate (PFPAT) successfully catalyzed the C-acylation of enol silyl ethers with acid chloride to produce various β-diketones in good yield. Similarly, C-acylation of ketene silyl acetals or ketene silyl thioacetals proceeded smoothly to provide also thermodynamically unfavorable α,α-dialkylated β-keto (thio)esters in good yield.
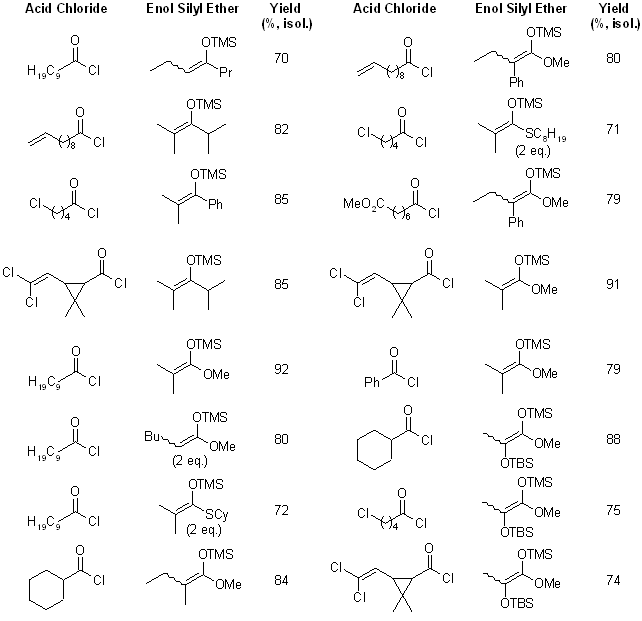
see article for more examples
A. Iida, S. Nakazawa, T. Okabayashi, A. Horii, T. Misaki, Y. Tanabe, Org. Lett., 2006, 8, 5215-5218.
Key Words
Claisen Condensation, Diketones, Ketocarboxylic Esters, Thioesters
ID: J54-Y2007-1350
