Silicon Tetrachloride Catalyzed Aza-Michael Addition of Amines to Conjugated Alkenes under Solvent-Free Conditions
Najmedin Azizi*, Roya Baghi, Hossein Ghafuri, Mohammad Bolourtchian, Mohammad Hashemi
*Chemistry and Chemical Engineering Research Center of Iran,
P.O. Box 14335-186, Tehran, Iran, Email: azizi ccerci.ac.ir
ccerci.ac.ir
N. Azizi, R. Baghi, H. Ghafuri, M. Boloutchian, M. Hashemi, Synlett, 2010, 379-382.
DOI: 10.1055/s-0029-1219195

Abstract
An efficient and very simple conjugate addition of aromatic and aliphatic amines to α,β-unsaturated carbonyl compounds under solvent-free conditions in the presence of catalytic amount of silicon tetrachloride gave the corresponding Michael adducts with very good yields.
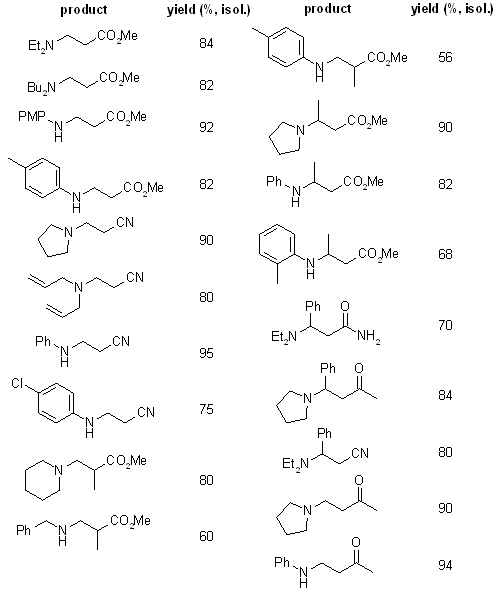
see article for more examples
LiClO4 Accelerated Michael addition of amines to α,β-unsaturated olefins under solvent-free conditions
N. Azizi, M. R. Saidi, Tetrahedron, 2004, 60, 383-387.
Key Words
aromatic amines, conjugate additions, silicon tetrachloride, β-aminocarbonyl compounds, Michael Addition
ID: J60-Y2010-0260
