Efficient Aqueous-Phase Heck Reaction Catalyzed by a Robust Hydrophilic Pyridine-Bridged Bisbenzimidazolylidene-Palladium Pincer Complex
Zhixun Wang, Xike Feng, Weiwei Fang, Tao Tu*
*Department of Chemistry, Fudan University, 220 Handan Road, 200433 Shanghai,
P. R. of China, Email: taotu fudan.edu.cn
fudan.edu.cn
Z. Wang, X. Feng, W. Fang, T. Tu, Synlett, 2011, 951-954.
DOI: 10.1055/s-0030-1259723
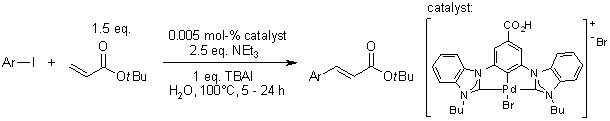
see article for more reactions
Abstract
An NHC-palladium pincer complex catalyzes aqueous Heck reactions with extremely low catalyst loading without obvious hydrolysis of products. The robust hydrophilic pyridine-bridged bisbenzimidazolylidene-palladium complex, that is used as the molecular catalyst, allows the conversion of a broad range of aryl iodides.
 see article for more examples
see article for more examples
Key Words
aqueous phase, green chemistry, benzimidazole, Heck reaction, Alkenylation, N-heterocyclic carbene, palladium
ID: J60-Y2011-0960
