A reductive amination of carbonyls with amines using decaborane in methanol
Jong Woo Bae, Seung Hwan Lee, Young Jin Cho, Cheol Min Yoon*
*Department of Life Science & Biotechnology, Graduate school of
Biotechnology, Korea University, 1, 5-Ka, Anam-Dong Sungbuk-Ku, Seoul, 136-701
Korea, Email: cmyoon korea.ac.kr
korea.ac.kr
J. W. Bae, S. H. Lee, Y. J. Cho, C. M. Yoon, J. Chem. Soc., Perkin Trans. 1, 2000, 145-146.
DOI: 10.39/a909506c

Abstract
Aldehydes and ketones were easily converted to the corresponding amines by the reaction of amines in methanol using decaborane (B10H14) at room temperature under nitrogen. The reaction is simple and efficient.
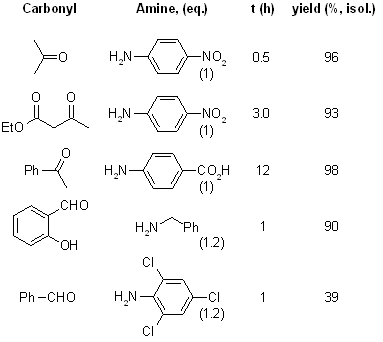 see article for more examples
see article for more examples
J. W. Bae, Y. J. Cho, S. H. Lee, C.-O. M. Yoon, C. M. Yoon, Chem. Commun., 2000, 1857-1858.
Reductive Etherification of Aromatic Aldehydes with Decaborane
S. H. Lee, Y. J. Park, C. M. Yoon, Tetrahedron Lett., 1999, 40, 6049-6050.
Key Words
reductive amination, decaborane
ID: JXX-Y2000-370
