Categories: C-C Bond Formation > Arenes >
Cyclizations
Name Reactions
Recent Literature

A cobalt-catalyzed neutral Diels-Alder reaction of dienes derived from aldehydes
with terminal and internal alkynes, and DDQ oxidation of the dihydroaromatic
intermediates leads to regiochemically enriched biphenyl, terphenyl, and
silyl-functionalized benzene derivatives in good to excellent yields.
G. Hilt, M. Danz, Synthesis, 2008,
2257-2263.

The cobalt-catalyzed Diels-Alder reaction of alkynyl pinacol boronic esters
with various dienes generates cycloadducts in very good regioselectivity. A reaction sequence (Diels-Alder reaction, Suzuki coupling, DDQ oxidation)
was successfully performed as a one pot operation without isolation of the
intermediates.
G. Hilt, K. I. Smolko, Angew. Chem. Int. Ed., 2003, 42,
2795-2797.

Intramolecular carbonyl allylation of α-prenyl β-arylketosulfones in the
presence of molecular sieves affords substituted benzenes in good yields via
Bi(OTf)3-mediated annulation followed by a sequential desulfonative
aromatization. The use of α-geranyl β-arylketosulfones enables the synthesis of tetrahydronaphthalenes.
M.-Y. Chang, Y.-C. Cheng, Y.-J. Lu, Org. Lett.,
2015,
17, 3142-3145.

A Mo-catalyzed intermolecular deoxygenative benzene-forming reaction of
readily available ynones and allylic amines provides a wide range of unsymmetric
and unfunctionalized 1,3-di- and 1,3,5-trisubstituted benzenes in good yield.
The synthetic potential of the method was further illustrated by synthetic
transformations, a scale-up synthesis, and derivatization of bioactive molecules.
Y.-Z. Yu, J. Bai, J.-M. Peng, J.-S. Yao, C.-X. Zhuo, J. Am. Chem. Soc.,
2023, 145, 8781-8787.

DBU mediates a [2 + 4] annulation of in situ activated α,β-unsaturated
carboxylic acids and α-cyano-β-methylenones to provide 1,3,5-trisubstituted
benzenes. The dual role of DBU as Brønsted base and nucleophilic Lewis base is
the key for the success of the reaction.
C.-L. Zhang, Z.-F. Zhang, Z.-H. Xia, Y.-F. Han, S. Ye, J. Org. Chem., 2018, 83,
12507-12513.

A N-heterocyclic carbene-catalyzed [2 + 4] annulation of α-bromoenals and
α-cyano-β-methylenones enables a direct and efficient approach to
1,3,5-trisubstituted benzenes. The reaction worked well for both aryl- and
alkylenones.
C.-L. Zhang, S. Ye, Org. Lett.,
2016, 18, 6408-6411.

An efficient base-catalyzed [3 + 3] oxidative aromatization of α,β-unsaturated
carbonyl compounds with dimethyl glutaconate under mild, metal-free conditions
affords substituted benzenes in high to excellent yields with oxygen as oxidant
and water as sole byproduct. In situ generation of the α,β-unsaturated carbonyl
compounds from aldehydes and ketones enables a more convenient tandem [3 + 2 +
1] aerobic oxidative aromatization reaction.
A. Diallo, Y.-L. Zhao, H. Wang, S.-S. Li, C.-Q. Ren, Q. Liu, Org. Lett., 2012,
14, 5776-5779.

A. Diallo, Y.-L. Zhao, H. Wang, S.-S. Li, C.-Q. Ren, Q. Liu, Org. Lett., 2012,
14, 5776-5779.

Highly Selective Diels-Alder Reactions of Directly Connected Enyne
Dienophiles
M. Dai, D. Sarlah, M. Yu, S. J. Danishefsky, G. O. Jones, K. N. Houk, J. Am. Chem. Soc., 2007,
129, 645-657.

Salicylic acid derivatives were prepared by Me3SiOTf-catalyzed [3
+ 3] cyclization of 1,3-bis(silyl enol ethers) with 1,1,3,3-tetramethoxypropane
under mild conditions in moderate yields.
M. Sher, T. H. T. Dang, Z. Ahmed, M. A. Rashid, C. Fischer, P. Langer, J. Org. Chem., 2007,
72, 6284-6286.
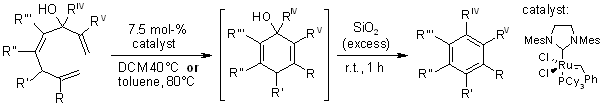
Various substituted phenol and benzene derivatives were prepared through
RCM-tautomerization and RCM-dehydration protocols without formation of
inseparable regioisomers.
K. Yoshida, S. Horiuchi, N. Iwadate, F. Kawagoe, T. Imamoto, Synlett, 2007,
1561-1562.

K. Yoshida, S. Horiuchi, N. Iwadate, F. Kawagoe, T. Imamoto, Synlett, 2007,
1561-1562.

A New Synthetic Approach to Phenol Derivatives: Use of Ring-Closing Olefin
Metathesis
K. Yoshida, T. Imamoto, J. Am. Chem. Soc., 2005, 127, 10470-10471.

Synthesis of Styrenes Using Ruthenium-Catalyzed Ring-Closing Enyne
Metathesis
K. Yoshida, Y. Shishikura, H. Takahashi, T. Imamoto, Org. Lett.,
2008,
10, 2777-2780.

A ring-closing olefin metathesis (RCM)/elimination sequence or an RCM/tautomerization
sequence of functionalized pyrrole precursors enabled the selective synthesis of
substituted indoles. The RCM/elimination sequence was also applied to double
ring closure to yield a substituted carbazole.
K. Yoshida, K. Hayashi, A. Yanagisawa, Org. Lett., 2011,
13, 4762-4765.

A AuCl-catalyzed, flexible synthesis of highly substituted, benzyl-protected
phenols unites enal/enones and benzyl allenyl ethers in a [3+3] fashion in two
steps, allowing excellent control of substitution at the benzene ring.
X. Huang, L. Zhang, Org. Lett., 2007,
9, 4627-4630.

A N-heterocycle carbene (NHC)-catalyzed dual Stetter cascade reaction
enables a coupling of β-nitrostyrene with phthalaldehyde under mild conditions
to furnish valuable aryl-naphthoquinones, whereas the reaction of
salicylaldehydes with β-nitrostyrene provides functionalized
dihydroisoflavanones.
R. N. Mitra, K. Show, D. Barman, S. Sarkar, D. K. Maiti, J. Org. Chem., 2019, 84,
42-52.

An N-heterocyclic carbene (NHC)-catalyzed intramolecular Stetter reaction
enables a transition-metal-free and mild cross-dehydrogenative coupling of
2-cinnamoyl benzaldehydes to provide 2-aryl naphthoquinones. The intramolecular
Stetter reaction is followed by an aerobic oxidation to reinstall the C-C double
bond.
A. Ghosh, A. Patra, S. Mukherjee, A. T. Biju, J. Org. Chem., 2019, 84,
1103-1110.

X. Huang, L. Zhang, Org. Lett., 2007,
9, 4627-4630.

A gold(I)-catalyzed intramolecular hydroarylation of (Z)-2-(enynyl)indoles
gives carbazoles in good yields. The requisite (Z)-2-(enynyl)indoles were
synthesized stereoselectively by trimethylgallium-promoted, Z-selective
Wittig olefination of N-alkylindole-2-carboxaldehydes with propargyl
ylides.
C. Praveen, P. T. Perumal, Synlett, 2011,
268-272.

A cationic rhodium(I)/BINAP complex-catalyzed decarboxylative [2 + 2 + 2]
cycloaddition of 1,6- and 1,7-diynes with commercially available vinylene
carbonate enables a new route to substituted phenols.
H. Hara, M. Hirano, K. Tanaka, Org. Lett., 2009,
11, 1337-1340.