Categories: C-S Bond Formation >
Related |
|
Synthesis of sulfides (thioethers) and derivatives
Recent Literature

The combination of trimethyl phosphate (TMP) and Ca(OH)2 enables a
mild and efficient heteroatom methylation in DMF, water, or under neat
conditions, at 80 °C or at room temperature. A series of O-, N-, and
S-nucleophiles, including phenols, sulfonamides, N-heterocycles, such as
9H-carbazole, indole derivatives, and 1,8-naphthalimide, and aryl/alkyl
thiols, are suitable substrates.
Y. Tang, B. Yu, Synthesis, 2022, 54,
2373-2390.

An efficient, indium triiodide-catalyzed substitution of the acetoxy group in
alkyl, benzyl, allyl, and propargyl acetates with thiosilanes provides access to
various thioethers.
Y. Nishimoto, A. Okita, M. Yasuda, A. Baba, Org. Lett., 2012,
14, 1846-1849.

S-Alkyl, S-aryl, and S-vinyl thiosulfate sodium salts (Bunte salts), which can
readily be prepared from sodium thiosulfate, react with Grignard reagents to
give sulfides in good yields. The reaction is amenable to a broad structural
array of Bunte salts and Grignard reagents. Importantly, this route to sulfides
avoids the use of malodorous thiol starting materials or byproducts.
J. T. Reeves, K. Camara, Z. S. Han, Y. Xu, H. Lee, C. A. Busacca, C. H.
Senanayaka, Org. Lett., 2014,
16, 1196-1199.

In three odorless methods for the thioarylation and thioalkylation of different
nitroarenes using alkyl halides (Br, Cl), triphenyltin chloride, and arylboronic
acids as the coupling partners, Na2S2O3·5H2O,
S8/KF, and S8/NaOH systems are found to be effective
sources of sulfur in the presence of copper salts. The methods offer use of
green solvents, inexpensive catalysts, and user-friendly starting materials.
A. Rostami, A. Rostami, A. Ghaderi, J. Org. Chem.,
2015,
80, 8694-8704.

A mild organophotoredox protocol enables the formation of Csp3-S/Se
bonds by reacting redox-active esters with thio/selenosulfonates. The reaction
offers an unprecedented broad substrate scope and wide functional group
tolerance.
Y. Dong, P. Ji, Y. Zhang, C. Wang, X. Meng, W. Wang, Org. Lett., 2020, 22,
9562-9567.

A Zn(OTf)2-catalyzed coupling of alcohols/phenols with unactivated
tertiary alkyl bromides and a Cu(OTf)2-catalyzed thiolation of unactivated
tertiary alkyl oxalates with thiols provide sterically hindered tertiary alkyl
ethers and thioethers.
Y. Gong, Z. Zhu, Q. Qian, W. Zong, H. Gong, Org. Lett., 2021, 23,
1005-1010.

A visible-light-promoted cross-coupling of
4-alkyl-1,4-dihydropyridines with thio-/selenium sulfonates under
transition-metal-free conditions provides sulfides or selenides in high yields
and chemoselectivity under mild transition-metal-free reaction conditions. Using
air as the atmosphere, the reaction provides sulfoxides.
J. Li, X.-E. Yang, S.-L. Wang, L.-L. Zhang, X.-Z. Zhou, S.-Y. Wang, S.-J. Ji,
Org. Lett., 2020, 22, 4908-4913.

Fluoroolefins-Mediated Deoxygenative Substitution of Alcohols: Chemo- and
Enantiospecific Construction of C-S, C-N, C-O, and C-Se Bonds
H. Li, L. Qin, J. Nie, H. Jiang, C. Zhu, Org. Lett., 2023, 25,
5431-5436.

A convenient thiol-ene reaction is performed under blue-light irradiation and catalyzed by photoactive
Lewis basic molecules such as acridine orange or naphthalene-fused N-acylbenzimidazole. It is believed that the process is initiated by a
proton-coupled electron transfer process within the complex between the thiol
and the Lewis basic catalyst.
V. V. Levin, A. D. Dilman, J. Org. Chem., 2019, 84,
8337-8343.

A reducing system combined with InBr3 and
1,1,3,3-tetramethyldisiloxane (TMDS) enables a direct thioetherification of
various aromatic carboxylic acids and thiols in a one-pot procedure, whereas a
system combined with InI3 and TMDS underwent thioetherification of
aliphatic carboxylic acids with thiols.
N. Sakai, T. Miyazaki, T. Sakamoto, T. Yatsuda, T. Moriya, R. Ikeda, T.
Konakahara, Org. Lett., 2012,
14, 4366-4369.

Low catalyst loading of Bi2O3 enables a nontoxic and
inexpensive photocatalytic initiation of a mild and selective anti-Markovnikov
hydrothiolation of olefins using visible light.
O. O. Fadeyi, J. J. Mousseau, Y. Feng, C. Allais, P. Nuhant, M. Z. Chen, B.
Pierce, R. Robinson, Org. Lett.,
2015,
17, 5756-5759.

Synthetically useful high-yielding, radical thiol-ene reactions can be initiated
by visible light irradiation in the presence of transition metal polypyridyl
photocatalysts in the presence of p-toluidine as a redox mediator that is
capable of catalyzing the otherwise inefficient photooxidation of thiols to the
key thiyl radical intermediate. Thus, co-catalytic oxidants can be important in
the design of synthetic reactions involving visible light photoredox catalysis.
E. L. Tyson, Z. L. Niemeyer, T. P. Yoon, J. Org. Chem., 2014,
79, 1427-1436.

A gold-catalyzed hydrothiolation of unactivated alkenes proceeds effectively to
give anti-Markovnikov-selective adducts in good yields and in a
regioselective manner.
T. Tamai, K. Fujiwara, S. Higashimae, A. Nomoto, A. Ogawa, Org. Lett.,
2016, 18, 2114-2117.

The use of visible-light-absorbing transition metal photocatalysts enables an
anti-Markovnikov hydrothiolation of olefins. Quenching of the photoexcited
Ru*(bpz)32 catalyst with a variety of thiols generates key
thiyl radical intermediates, that form adducts with a wide variety of olefins in
excellent yield.
E. L Tyson, M. S. Ament, T. P. Yoon, J. Org. Chem., 2013,
78, 2046-2050.

The anti-Markovnikov addition of thiols to alkenes using CeCl3 as
catalyst leads to products in very good yields. The reaction occurred under
solvent-free conditions at room temperature.
C. C. Silveira, S. R. Mendes, F. M. Líbero, Synlett, 2010,
790-792.

A highly selective anti-Markovnikov addition of thiols to unactivated alkenes in
water at room temperature without any additive is a very simple and efficient
method for the synthesis of linear thioethers.
B. C. Ranu, T. Mandal, Synlett, 2007, 925-928.

A nickel-catalyzed C-S reductive cross-coupling of alkyl halides with
arylthiosilanes provides alkyl aryl thioethers. This reaction offers exquisite
chemoselectivity, excellent tolerance of diverse functional groups, and wide
applications for late-stage modification of biologically relevant molecules.
Y.-Z. Yang, Y. Li, G.-F. Lv, D.-L. He, J.-H. Li, Org. Lett.,
2022, 24, 5116-5119.

The Rh-catalyzed hydrothiolation of 1,3-dienes affords either allylic or
homoallylic sulfides. Regioselectivity is dictated by the choice of counterion
associated with the Rh center. Coordinating counterions, such as Cl-,
favor neutral Rh complexes in which the diene binds η2 to afford
homoallylic sulfides.
X.-H. Yang, R. T. Davison, S.-Z. Nie, F. A. Cruz, T. M. McGinnis, V. M. Dong, J. Am. Chem. Soc.,
2019,
141, 3006-3013.

A wide range of ynol ethers can be prepared via displacement at an sp center.
The same protocol can be applied to the synthesis of synthetically useful
thioynol ethers. This reaction, which generates highly functionalized,
heteroatom-substituted alkynes, involves radical intermediates.
V. J. Gray, J. Cuthbertson, J. D. Wilden, J. Org. Chem., 2014,
79, 5869-5874.

A chiral iron(III)-salen complex catalyzes a regioselective, enantioselective
conjugate addition of thiols to acyclic α,β,γ,δ-unsaturated dienones at the δ
carbon to provide δ-thia-α,β-unsaturated ketones in high yield and
enantioselectivity. A model providing an explanation for the regio- and
stereoselection is proposed.
S. Shaw, J. D. White, Org. Lett.,
2015, 17, 4564-4567.

A new synthetic method for the preparation of potassium organotrifluoroborates
through nucleophilic substitution of potassium bromo- and
iodomethyltrifluoroborates is described. Potassium halomethyltrifluoroborates
have been prepared via in situ reaction of n-BuLi with dibromo- and
diiodomethane, respectively, in the presence of trialkyl borates, followed by
treatment with KHF2.
G. A. Molander, J. Ham, Org. Lett.,
2006, 8, 2031-2034.
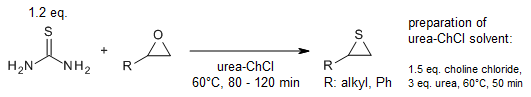
A general, straightforward and odourless ring-opening reaction allows the
preparation of β-hydroxy sulfides from in situ generated S-alkylisothiouronium
salts in urea-choline chloride-based deep eutectic solvent (DES). The reaction
of epoxides with thiourea in DES yields the corresponding thiiranes.
N. Azizi, Z. Yadollahy, A. Rahimzadeh-oskooee,
Synlett, 2014, 25, 1085-1088.

A highly regioselective chlorothiolation of alkenes with sulfonyl chlorides
is compatible with a variety of functional groups and can be scaled up to the
gram scale. The employment of readily available reactants and mild reaction
conditions makes this process very practical.
J. Wei, S. Liang, L. Jiang, Y. Mumtaz, Y.-b. Yi, J. Org. Chem., 2020, 85,
977-984.

A N-heterocyclic carbene mediates a direct dehydroxytrifluoromethylthiolation of alcohols
with a hypervalent iodine(III) reagent to provide CF3S-containing compounds. This method
shows excellent stereospecificity and chemoselectivity to give a product with
clean inversion of the stereochemical configuration.
X.-G. Yang, J.-J. Li, F.-H. Du, Y.-X. Cheng, C. Zhang, Org. Lett., 2023, 25,
2964-2969.

Epoxides can be opened under neutral conditions with alcohols and thiols in the
presence of a catalytic amount of erbium(III) triflate, affording the
corresponding β-alkoxy alcohols and β-hydroxy sulfides in high yields. In water,
epoxide ring opening occurs to produce the corresponding diols in good yields.
R. Dalpozzo, M. Nardi, M. Oliverio, R. Paonessa, A. Procopio, Synthesis,
2009, 3433-3438.

An NH4I-promoted and H2O-controlled intermolecular
difunctionalization of alkenes provides bis-methylsulfanes and β-hydroxysulfides
via methylthiyl radical addition to the double bond to give a carbon-centered
radical, which immediately cyclizes to a thiiranium ion, followed by combination
with H2O to afford β-hydroxysulfides. In the absence of water,
1,2-disulfenylation takes place.
R. He, X. Chen, Y. Li, Q. Liu, C. Liao, L. Chen, Y. Huang, J. Org. Chem., 2019, 84,
8750-8758.

A sulfoxide reduction/C-S bond metathesis cascade between sulfoxides and
alkyl bromides in an ionic liquid provides high-value sulfides without the use
of any catalysts or bases. In this cascade, classical Kornblum oxidation is
employed to reduce sulfoxides with alkyl bromides. This protocol features high
functional tolerance, mild conditions, promising scalability, and a sustainable
solvent.
C. Liu, D. Chen, Y. Fu, F. Wang, J. Luo, S. Huang,
Org. Lett., 2020, 22, 5701-5705.

A sulfoxide reduction/C-S bond metathesis cascade between sulfoxides and
alkyl bromides in an ionic liquid provides high-value sulfides without the use
of any catalysts or bases. In this cascade, classical Kornblum oxidation is
employed to reduce sulfoxides with alkyl bromides. This protocol features high
functional tolerance, mild conditions, promising scalability, and a sustainable
solvent.C. Liu, D. Chen, Y. Fu, F. Wang, J. Luo, S. Huang,
Org. Lett., 2020, 22, 5701-5705.

A gold-catalyzed reaction of sulfonium and selenium ylides with
aryldiazoacetates provides products of a [2,3]-sigmatropic rearrangement in high
yields. A reaction of aryl allyl anilines delivers exclusively C-H functionalized products.
F. He, S. Jana, R. M. Koenigs, J. Org. Chem., 2020, 85,
11882-11891.

An environmentally friendly and operationally simple DBU-catalyzed aerobic
cross-dehydrogenative coupling (CDC) reaction provides thiolated products of (hetero)aryl
acetates, (hetero)aryl ketones, and indoles in good yields.
X. Jia, X. Ma, W.-F. J.-Q. Zhang, Y. Zhao, B. Guo, L. Tang, Y.-Y. Yang, J. Org. Chem., 2022, 87,
16492-16505.

A direct difunctionalization protocol of alkenes with nitriles and thiols under
metal-free synthesis conditions provides various β-acetamido sulfides with very
good yields simply by using inexpensive molecular iodine as a catalyst, DMSO as
a mild oxidant, and readily available thiols as thiolating reagents.
H. Cui, X. Liu, W. Wei, D. Yang, C. He, T. Zhang, H. Wang, J. Org. Chem.,
2016,
81, 2252-2260.

In a direct and efficient method for the acetamidosulfenylation reaction of
alkenes, NaI was used as a catalyst, DMSO as the oxidant, nitriles as both the
solvent and nucleophiles and stable, readily available Bunte salts as thiolating
reagents. The reactions were carried out under mild conditions to provide
β-acetamido sulfides in good yields. Moreover, the reaction can be performed
with alcohols as nucleophiles.
R. Zhang, Z. Yan, D. Wang, Y. Wang, S. Lin,
Synlett, 2017, 28, 1195-1200.

Aziridines undergo an efficient, mild and regioselective ring opening with
various thiols in the presence of 5 mol% bismuth triflate to afford the
corresponding β-aminosulfides in excellent yields.
J. S. Yadav, B. V. S. Reddy, G. Baishya, P. V. Reddy, S. J. Harshavardhan,
Synthesis,
2004, 1854-1858.

Sterically encumbered chiral pyrrolidine derivatives are highly efficient
organocatalysts for the direct enantioselective α sulfenylation of aldehydes
using an electrophilic sulfur source.
M. Marigo, T. C. Wabnitz, D. Fielenbach, K. A. Jorgensen, Angew. Chem. Int.
Ed.,
2005,
44, 794-797.

By rendering the α-position of amides electrophilic through a mild and
chemoselective umpolung transformation, a broad range of widely available
oxygen, nitrogen, sulfur, and halogen nucleophiles can be used to generate
α-functionalized amides.
C. R. Gonçalves, M. Lemmerer, C. J. Teskey, P. Adler,
D. Kaiser, B. Maryasin, L. González, N. Maulide, J. Am. Chem. Soc.,
2019, 141, 18437-18443.

An efficient copper-catalyzed carbenoid insertion reaction of α-diazo carbonyl
compounds into Si-H and S-H bonds provides a broad range of α-silylesters and
α-thioesters in high yields using 5 mol% of a simple copper(I) salt as catalyst.
In addition, α-diazoketones can be converted to α-silylketones in moderate
yields.
H. Keipour, A. Jalba, L. Delage-Laurin, T. Ollevier, J. Org. Chem.,
2017, 82, 3000-3010.

A regio and anti-selective copper-catalyzed 1,2-hydroxysulfenylation of
alkenes can be carried out by the use of disulfides and acetic acid. Reoxidation
of intermediate sulfides by oxygen enables the use of both organosulfide groups
of the disulfides.
N. Taniguchi, J. Org. Chem., 2006, 71, 7874-7876.

A transition-metal-free, direct, and efficient acetamidosulphenylation reaction
of alkenes using nitriles as the nucleophiles offers a broad substrate scope and
high regioselectivity and provides straightforward access to acetamidosulfide
derivatives in good yields via a radical process.
Y. Zheng, Y. He, G. Rong, X. Zhang, Y. Wang, K. Dong, X. Xu, J. Mao, Org.
Lett.,
2015,
17, 5444-5447.

p-Toluenesulfonic acid efficiently catalyzes direct nucleophilic
substitutions of the hydroxy groups of propargylic alcohols with a large variety
of carbon- and heteroatom-centered nucleophiles. Reactions can be conducted
under mild conditions and in air without the need for dried solvents.
R. Sanz, A. Martinez, J. M. Alvarez-Gutierrez, F. Rodriquez, Eur. J. Org.
Chem., 2006, 1383-1386.

Under basic conditions, nucleophilic addition to gem-difluorostyrenes
generates an anionic intermediate that can undergo facile elimination of
fluoride to generate α-fluorovinylthioethers. An acid-based catalyst system
facilitates simultaneous nucleophilic addition and protonation of the unstable
intermediate to afford the desired α,α-difluoroalkylthioethers in high
selectivity and good yields.
J. P. Sorrentino, D. L. Orsi, R. A. Altman, J. Org. Chem., 2021, 86,
2297-2311.