Categories: C=O Bond Formation > Synthesis of ketones >
Synthesis of ketones by cleavage of alkenes
| Related |
|
|
Name Reactions
Recent Literature

The OsO4-catalyzed direct oxidation of olefins via the
carbon-carbon cleavage of an osmate ester by the action of oxone allows the
preparation of ketones or carboxylic acids in high yields. This method
should be applicable as an alternative to ozonolysis.
B. R. Travis, R. S. Narayan, B. Borhan, J. Am. Chem. Soc., 2002, 124, 3824-3825.

A light-driven, Mn-catalyzed protocol for the selective oxidation of alkenes
to carbonyls under 1 atm of O2 provides ketones and aldehydes under
clean, mild conditions. Aromatic as well as various nonactivated aliphatic
alkenes could be oxidized with a first row, biorelevant metal catalyst. Moreover,
the protocol shows a very good functional group tolerance.
Z. Huang, R. Guan, M. Shanmugam, E. L. Bennett, C. M. Robertson, A.
Brookfield, E. J. L. MeInnes, J. Xiao, J. Am. Chem. Soc.,
2021, 143, 10005-10013.

CsPbBr3 nanocrystals catalyze a facile visible-light-driven
oxidative cleavage of C=C bonds to the corresponding carbonyls. This catalytic
system was applicable to a wide range of terminal and internal alkenes.
Q. Fan, H. Zhang, D. Liu, C. Yan, H. Zhu, Z. Xie, Z. Le, J. Org. Chem., 2023, 88,
7391-7400.

N-hydroxyphthalimide (NHPI) catalyzes a metal-free, aerobic oxidative
cleavage of olefins. This methodology avoids the use of toxic metals or
overstoichiometric amounts of traditional oxidants, showing good economical and
environmental advantages. Based on the experimental observations, a plausible
mechanism is proposed.
R. Lin, F. Chen, N. Jiao, Org. Lett., 2012,
14, 4158-4161.

Sodium benzene sulfinate catalyzed a visible-light-driven aerobic oxidative
cleavage of olefins to provide the corresponding aldehydes and ketones under
transition-metal-free conditions. Notably, α-halo-substituted styrenes proceeded
with photoinduced oxidation to finally afford α-halo-acetophenones with halogen
migration.
Y.-X. Chen, J.-T. He, M.-C. Wu, Z.-L. Liu, K. Tang, P.-J. Xia, K. Chen, H.-Y.
Xiang, X.-Q. Chen, H. Yang, Org. Lett.,
2022, 24, 3920-3925.
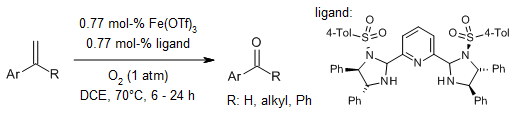
A mild and operationally simple protocol for the selective aerobic oxidation of
aromatic olefins to carbonyl compounds is catalyzed by a Fe(III) species bearing
a pyridine bisimidazoline ligand at 1 atm of O2. The method cleaves
α- and β-substituted styrenes to afford benzaldehydes and aromatic ketones in
high yields with excellent chemoselectivity and very good functional group
tolerance.
A. Gonzalez-de-Castro, J. Xiao, J. Am. Chem. Soc., 2015,
137, 8206-8218.

In a 2,2-azobis(isobutyronitrile)-catalyzed oxidative cleavage of gem-disubstituted
alkenes with molecular oxygen as the oxidant, carbonyl compounds were obtained
as the desired products in high yield under mild conditions.
G.-Z. Wang, X.-L. Li, J.-J. Dai, H.-J. Xu, J. Org. Chem., 2014,
79, 7220-7225.

The use of nitroarenes as oxygen transfer reagents enables an anaerobic
cleavage of a wide range of alkenes possessing oxidatively sensitive
functionalities into carbonyl compounds under visible light irradiation. This
approach serves as a safe and practical alternative to mainstream oxidative
cleavage protocols, such as ozonolysis and the Lemieux-Johnson reaction.
D. E. Wise, E. S. Gogarnoiu, A. D. Duke, J. M. Paolillo, T. L. Vacala, W. A.
Hussain, M. Parasram, J. Am. Chem. Soc.,
2022, 144, 15437-15442.

A gold(I)-catalyzed oxidative cleavage of alkenes with tert-butyl
hydrogenperoxide (TBHP) as the oxidant in the presence of neocuproine
afforded ketones or aldehydes as products.
D. Xing, B. Guan, G. Cai, Z. Fang, L. Yang, Z. Shi, Org. Lett.,
2006, 8, 693-696.

Specific oxidation protocols have been developed for the cleavage of styrenes,
aliphatic olefins, and terminal aliphatic olefins to carbonyl compounds with
ruthenium trichloride as catalyst. Olefins that are not fully substituted are
converted to aldehydes rather than carboxylic acids.
D. Yang, C. Zhang, J. Org. Chem., 2001, 66, 4814-4818.

A catalytic amount of a composite material, RuO2/BaTi4O9,
in combination with NaIO4 in EtOAc-H2O has been shown to
efficiently cleave alkenes, affording ketones, aldehydes and/or carboxylic acids
in high yields.
H. Okumoto, K. Ohtsuko, S. Banjoya, Synlett, 2007,
3201-3205.

Osmium tetroxide has been microencapsulated in a polyurea matrix. These
microcapsules have been effectively used as recyclable catalysts in the
dihydroxylation and the oxidative cleavage of olefins.
S. V. Ley, C. Ramarao, A.-L. Lee, N. Ostergaard, S. C. Smith, I. M. Shirley,
Org. Lett., 2003, 5, 185-187.

An iron-sulfur complex formed by the simple mixture of FeCl3 with
S3•- generated in situ from K2S mediates an
aerobic oxidation of terminal alkenes under an atmosphere of O2 (balloon).
The reaction could proceed on a gram scale, expanding the application of S3•-
in organic synthesis.
J.-J. Ai, B.-B. Liu, J. Li, F. Wang, C.-M. Huang, W. Rao, S.-Y. Wang, Org. Lett., 2021, 23,
4705-4709.

The use of PhI(OAc)2 in dichloromethane enables a clean oxidative
cleavage of 1,2-diols to aldehydes. In the presence of OsO4 as
catalyst, NMO and 2,6-lutidine, olefinic bonds can be cleaved in acetone/water
to yield the corresponding carbonyl compounds.
K. C. Nicolaou, V. A. Adsool, C. R. H. Hale, Org. Lett., 2010,
12, 1552-1555.

The oxidative cleavage of C=C bonds adjacent to aryl and alkyl moieties was
efficiently achieved with monoacetylated bishydroperoxides. Base-mediated
fragmentation of monoacetylated bishydroperoxides generates singlet molecular
oxygen as active oxidant in situ. All the reactions furnished the respective
carbonyl compounds in good yields at room temperature within short reaction
times.
D. Azarifar, Z. Najminejad, Synlett, 2013, 24, 1377-1382.