Categories: Synthesis of N-Heterocycles >
Synthesis of pyrimidines
| Related: |
|
Recent Literature

An oxidative annulation involving anilines, aryl ketones, and DMSO as a
methine (=CH−) equivalent promoted by K2S2O8
provides 4-arylquinolines, whereas activation of acetophenone-formamide
conjugates enables the synthesis of 4-arylpyrimidines.
S. D. Jadhav, A. Singh, Org. Lett.,
2017, 19, 5673-5676.

A ZnCl2-catalyzed three-component coupling reaction allows the
synthesis of various 4,5-disubstituted pyrimidine derivatives in a single step
from functionalized enamines, triethyl orthoformate, and ammonium acetate. The
procedure can be successfully applied to the efficient synthesis of mono- and
disubstituted pyrimidine derivatives, using methyl ketone derivatives instead of
enamines.
T. Sasada, F. Kobayashi, N. Sakai, T. Konakahara, Org. Lett., 2009,
11, 2161-2164.

An operationally simple, regioselective reaction of ketones, aldehydes, or
esters with amidines in the presence of TEMPO and an in situ prepared recyclable
iron(II)-complex provides various pyrimidine derivatives with broad functional
group tolerance. The reactions are likely to proceed through a TEMPO
complexation/enamine addition/transient α-occupation/β-TEMPO
elimination/cyclization sequence.
X.-Q. Chu, W.-B. Cao, X.-P. Xu, S.-J. Ji, J. Org. Chem.,
2017, 82, 1145-1154.

A base-promoted intermolecular oxidation C-N bond formation of allylic C(sp3)-H
and vinylic C(sp2)-H of allyllic compounds with amidines enables the
smooth formation of polysubstituted pyrimidines in the presence of O2
as the sole oxidant. This protocol features protecting group free nitrogen
sources, good functional group tolerance, high atom economy, and environmental
advantages.
W. Guo, C. Li, J. Liao, F. Ji, D. Liu, W. Wu, H. Jiang, J. Org. Chem.,
2016,
81, 5538-5546.

A Cu-catalyzed and 4-HO-TEMPO-mediated [3 + 3] annulation of commercially
available amidines with saturated ketones enables an efficient and facile
synthesis of structurally important pyrimidines via a cascade reaction of
oxidative dehydrogenation/annulation/oxidative aromatization.
J.-L. Zhang, M.-W. Wu, F. Chen, B. Han, J. Org. Chem.,
2016, 81, 11994-12000.

A copper-catalyzed cyclization of ketones with nitriles enables a facile,
general, and economical synthesis of diversely functionalized pyrimidines under
basic conditions. The reaction shows broad substrate scope and tolerates many
important functional groups.
L. Su, K. Sun, N. Pan, L. Liu, M. Sun, J. Dong, Y. Zhou, S.-F. Yin, Org. Lett.,
2018, 20, 3399-3402.

An efficient, facile, and eco-friendly synthesis of pyrimidine derivatives
involves an oxidative [3 + 2 + 1] three-component annulation of amidines,
ketones, and N,N-dimethylaminoethanol as the one carbon source. The
reaction tolerates many important functional groups.
Z. Qin, Y. Ma, F. Li, J. Org. Chem., 2021, 86,
13734-13743.
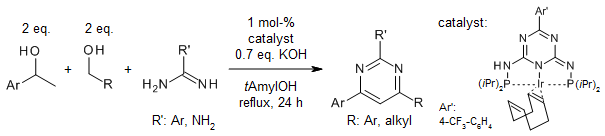
A regioselective, iridium-catalyzed multicomponent synthesis of pyrimidines from
amidines and up to three (different) alcohols proceeds via a sequence of
condensation and dehydrogenation steps. While the condensation steps deoxygenate
the alcohol components, the dehydrogenations lead to aromatization. PN5P-Ir-pincer
complexes catalyze this sustainable multicomponent process most efficiently.
N. Deibl, K. Ament, R. Kempe, J. Am. Chem. Soc., 2015,
137, 12804-12807.

A NaOH catalyzed rearrangement of propargylic hydroxylamines allows a highly
stereoselective access to Cbz-protected β-enaminones. A subsequent synthesis of
pyrimidines shows the synthetic potential of these β-enaminones.
E. Gayon, M. Szymczyk, H. Gérard, E. Vrancken, J.-M. Campagne, J. Org. Chem., 2012,
77, 9205-9220.

The direct condensation of cyanic acid derivatives with N-vinyl/aryl
amides affords the corresponding C4-heteroatom substituted pyrimidines. The use
of cyanic bromide and thiocyanatomethane in this chemistry provides versatile
azaheterocycles poised for further derivatization.
O. K. Ahmad, M. D. Hill, M. Movassaghi, J. Org. Chem., 2009,
74, 8460-8463.

A novel and efficient synthesis of pyrimidine from β-formyl enamide involves
samarium chloride catalysed cyclisation of β-formyl enamides using urea as
source of ammonia under microwave irradiation.
M. G. Barthakur, M. Borthakur, P. Devi, C. J. Saikia, A. Saikia, U. Bora, A.
Chetia, R. C. Boruah, Synlett, 2007, 223-226.

The coupling of acid chlorides with terminal alkynes using one equivalent of
triethylamine under Sonogashira conditions followed by subsequent addition
of amines or amidinium salts to the intermediate alkynones allows a
straightforward access to enaminones and pyrimidines under mild conditions
and in excellent yields.
A. S. Karpov, T. J. J. Müller, Synthesis, 2003, 2815-2826.

A single-step conversion of various N-vinyl and N-aryl amides
to the corresponding pyrimidine and quinazoline derivatives involves amide
activation with 2-chloropyridine and trifluoromethanesulfonic anhydride
followed by nitrile addition into the reactive intermediate and
cycloisomerization.
M. Movassaghi, M. D. Hill, J. Am. Chem. Soc., 2006, 128, 14254-14255.

Ultrasound irradiation promoted the cyclocondensation of β-keto esters and
amidines in good to excellent yields to form highly substituted 4-pyrimidinols.
A subsequent ultrasound-promoted tosylation followed by a Suzuki-Miyaura
cross-coupling provides 4-arylpyrimidines.
M. Vidal, M. García-Arriagada, M. C. Rezende, M. Domínguez, Synthesis, 2016,
48, 4246-4252.

Methyl 1,2,3-triazine-5-carboxylate reacts with alkyl and aryl amidines at
room temperature at remarkable rates (<5 min, 0.1 M in CH3CN) nearly
10000-fold faster than that of unsubstituted 1,2,3-triazine to provide product
pyrimidines in high yields. C4 Methyl substitution of the 1,2,3-triazine had
little effect on the rate of the reaction, whereas C4/C6 dimethyl substitution
slowed the reaction.
R. E. Quiñones, Z.-C. Wu, D. L. Boger, J. Org. Chem., 2021, 86,
13465-13474.

A method for the synthesis of 2-substituted pyrimidine-5-carboxylic esters
is described. The sodium salt of 3,3-dimethoxy-2-methoxycarbonylpropen-1-ol
has been found to react with a variety of amidinium salts to afford the
corresponding 2-substituted pyrimidine-5-carboxylic esters.
P. Zhichkin, D. J. Fairfax, S. A. Eisenbein, Synthesis, 2002, 720-722.

TFA-catalyzed inverse electron demand Diels-Alder (IEDDA) reactions of
electron-deficient 1,3,5-triazines and electron-deficient aldehydes/ketones
provide highly functionalized pyrimidines as products in good yields. The
reactions involve a cascade of stepwise inverse electron demand
hetero-Diels-Alder (ihDA) reactions, followed by retro-Diels-Alder (rDA)
reactions and elimination of water. An acid is required for both ihDA and rDA
reactions.
K. Yang, Q. Dang, P.-J. Cai, Y. Gao, Z.-X. Yu, X. Bai, J. Org. Chem.,
2017, 82, 2336-2344.

A metal-free method provides a very practical and efficient approach to
2,6-disubstituted 4-fluoropyrimidines in very good yields from readily
accessible α-CF3 aryl ketones and different amidine hydrochlorides
under mild conditions.
F. Liu, X. Zhang, Q. Qian, C. Yang, Synthesis, 2020, 52,
273-280.

The reaction of trifluorinated 2-bromoenones with aryl- and alkylamidines
provides trifluoromethylated pyrimidines in very good yields via an aza-Michael
addition-intramolecular cyclization-dehydrohalogenation/dehydration cascade
reaction. This strategy offers high selectivity and mild reaction conditions.
A. R. Romanov, A. Yu. Rulev, A. V. Popov, E. V. Kondrashov, S. V. Zinchenko, Synthesis, 2020, 52,
1512-1522.

An easy to prepare, tridentate arylazo pincer iron complex catalyzes an eco-friendly construction of trisubstituted pyrimidines under mild
aerobic conditions via dehydrogenative functionalization of alcohols with
alkynes and amidines.
R. Mondal, G. Chakraborty, A. K. Guin, S. Sarkar, N. D. Paul, J. Org. Chem., 2021, 86,
13186-13197.

An efficient reaction of readily available substituted
2-benzylidenemalononitriles and substituted benzamidines provides
2-aryl-5-benzylpyrimidine-4,6-diamines in good yields under simple reaction
conditions. This approach also enables some modifications of structurally
complex bioactive molecules.
C. Wu, X. Bian, L. Wang, Y. Zhang, C. Wang, Synthesis, 2023,
55,
457-464.

An array of tetrasubstituted saturated fused pyrimidines has been synthesized
through a simple and efficient one-pot operation. The strategic utilization of
the N-PMB group enabled the construction of a broad range of N-vinyl
tertiary enamide starting materials. This stands as a flexible approach to
functionalized pyrimidines with the capability of manipulating either ketone,
acid chloride, or nitrile reaction partners.
A. A. Estrada, J. P. Lyssikatos, F. St-Jean, P. Bergeron, Synlett, 2011,
2387-2391.