Categories: Synthesis of S-Heterocycles >
Synthesis of thiophenes
Name Reactions
Paal-Knorr Thiophene Synthesis
Recent Literature

Low catalyst loading of a bis(alkoxo)palladium complex enables an efficient
phosphine-free direct C-H arylation of thiophenes at C2. The developed synthetic
method couples aryl or heteroaryl bromides with thiophenes bearing
electron-donating or electron-withdrawing groups and other heterocyclic moieties
such as benzothiophene, benzofuran, and pyrrole with very good yields.
Y. Li, J. Wang, M. Huang, Z. Wang, Y. Wu, Y. Wu, J. Org. Chem., 2014,
79, 2890-2897.

A metal-free dehydration and sulfur cyclization of alkynols with elemental
sulfur (S8) or EtOCS2K provides a variety of substituted
thiophenes in good yields. The reaction is initiated by the base-free generation of a
trisulfur radical anion (S3•-) and its addition to
alkynes.
J. Li, Y. Liu, Z. Chen, J. Li, j. Li, X. Ji, L. Chen, Y. Huang, Q. Liu, Y.
Li, J. Org. Chem., 2022, 87,
3555-3566.
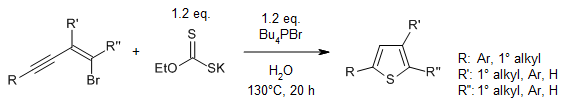
In an environmentally sustainable strategy for the chemoselective
heterocyclization of bromoenynes through a sulfuration/cyclization process,
inexpensive and safe EtOCS2K is used as a thiol surrogate and
tetrabutylphosphonium bromide and H2O as a mixed solvent. This
transition-metal-free reaction provides a range of substituted thiophenes in
good yields.
G. Huang, J. Li, J. Li, J. Li, M. Sun, P. Zhou, L. Chen, Y. Huang, S. Jiang,
Y. Li, J. Org. Chem., 2020, 85, 13037-13049.
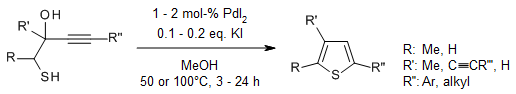
Various readily available 1-mercapto-3-yn-2-ols 5 were conveniently converted
into the corresponding thiophenes in good to high yields in MeOH as solvent in
the presence of PdI2 as catalyst and KI as additive. The use of
BmimBF4 as solvent enables the recycling of the catalyst.
B. Gabriele, R. Mancuso, L. Veltri, V. Maltese, G. Salerno, J. Org. Chem., 2012,
77, 9905-9909.

The interaction between elemental sulfur and NaOtBu enables a facile
single-step protocol for the synthesis of thiophene derivatives from 1,3-diynes.
A plausible mechanism based on EPR experiments revealed that the trisulfur
radical anion acts as a key intermediate of this process.
G. Zhang, H. Yi, H. Chen, C. Bian, C. Liu, A. Lei, Org. Lett.,
2014,
16, 6156-6159.

Trithiocarbonate anions (CS32-) can be generated in
situ from CS2 and KOH in dimethyl sulfoxide and used as a novel S2-
synthon for the synthesis of 2,5-disubstituted thiophenes from 1,3-butadiynes.
In addition, this system was employed for a metal-free synthesis of
2-substituted benzo[b]thiophenes from 2-haloalkynyl (hetero)arenes.
D. B. Paixão, D. S. Rampon, H. D. Salles, E. G. O. Soares, F. N. Bilheri, P.
H. Schneider, J. Org. Chem., 2020, 85,
12922-12934.

The reaction of substituted buta-1-enes with potassium sulfide enables an atom
economical, and transition-metal-free synthesis of thiophenes via cleavage of
multiple C-H bonds. Moreover, the strategy can also be used for the synthesis of
thiophenes from 1,4-diaryl-1,3-dienes.
L. Chen, H. Min, W. Zeng, X. Zhu, Y. Liang, G. Deng, Y. Yang, Org. Lett.,
2018, 20, 7392-7395.

The reaction of substituted buta-1-enes with potassium sulfide enables an atom
economical, and transition-metal-free synthesis of thiophenes via cleavage of
multiple C-H bonds. Moreover, the strategy can also be used for the synthesis of
thiophenes from 1,4-diaryl-1,3-dienes.
L. Chen, H. Min, W. Zeng, X. Zhu, Y. Liang, G. Deng, Y. Yang, Org. Lett.,
2018, 20, 7392-7395.

Copper-catalyzed tandem S-alkenylation of potassium sulfide with
1,4-diiodo-1,3-dienes enables an efficient synthetic approach to variously
substituted thiophenes.
W. You, X. Yan, Q. Liao, C. Xi, Org. Lett., 2010,
12, 3930-3933.

In a simple, regiocontrolled, and transition-metal-free approach to access
exclusively 3-borylated thiophene derivatives, commercially
available B-chlorocatecholborane acts as a carbophilic Lewis
acid to activate the alkyne in readily synthesized (Z)-organylthioenyne
substrates. A formal thioboration and
subsequent sulfur dealkylation provide 3-borylated
thiophenes in good yields.
H. B. Abed, S. A. Blum, Org. Lett.,
2018, 20, 6673-6677.

A mild, metal-free and base-promoted thioannulation of Morita-Baylis-Hillman
acetates of acetylenic aldehydes with potassium thioacetate to yield substituted
thiophenes involves a tandem allylic substitution/deacetylative 5-exo-dig-thiocycloisomerization.
The obtained products provide an entry to 4H-thieno[3,2-c]chromene
and thieno[3,2-c]dihydroquinoline.
C. R. Reddy, R. R. Valleti, N. D. Reddy, J. Org. Chem., 2013,
78, 6495-6502.

An efficient one-step method for the synthesis of highly substituted
thiophenes from thiomorpholides and α-haloketones was developed. The
mechanism is discussed.
F. Matloubi Moghaddam, H. Zali Bionee, Tetrahedron, 2004, 60,
6085-6089.

An efficient and highly versatile microwave-assisted Paal-Knorr condensation
of various 1,4-diketones gave furans, pyrroles and thiophenes in good yields.
In addition, transformations of the methoxycarbonyl moiety, such as Curtius
rearrangement, hydrolysis to carboxylic acid, or the conversion into amine
by reaction with a primary amine in the presence of Me3Al, are
described.
G. Minetto, L. F. Raveglia, A. Sega, M. Taddei, Eur. J. Org. Chem., 2005,
5277-5288.

Thionation of amides, 1,4-diketones, N-(2-oxoalkyl)amides, and N,N'-acylhydrazines
with the use of a fluorous Lawesson's reagent leads to thioamides, thiophenes,
1,3-thiazoles, and 1,3,4-thiadiazoles in high yields. The isolation of the final products is achieved in most cases by a
simple filtration.
Z. Kaleta, B. T Makowski, T. Soos, R. Dembinski, Org. Lett.,
2006, 8, 1625-1628.

Synthesis of 2-Alkoxy-Substituted Thiophenes, 1,3-Thiazoles, and Related S-Heterocycles via Lawesson's Reagent-Mediated Cyclization under Microwave
Irradiation: Applications for Liquid Crystal Synthesis
A. A. Kiryanov, P. Sampson, A. J. Seed, J. Org. Chem., 2001,
66, 7925-7929.

A direct iodocyclization of 1-mercapto-3-yn-2-ols derivatives enables the
synthesis of 3-iodothiophenes. Various substrates were smoothly converted into
the corresponding 3-iodothiophene derivatives in good yields by reaction with
molecular iodine in the presence of NaHCO3 at room temperature in
MeCN as the solvent.
B. Gabriele, R. Mancuso, G. Salerno, R. C. Larock, J. Org. Chem., 2012,
77, 7640-7645.

A rhodium-catalyzed transannulation reaction between 1,2,3-thiadiazoles and
alkynes enables a highly efficient and regioselective synthesis of highly
substituted thiophenes via intermediacy of a previously unknown Rh thiavinyl
carbene.
D. Kurandina, V. Gevorgyan, Org. Lett., 2016, 18,
1804-1807.

Ynone trifluoroborate salts undergo a base-promoted condensation reaction with
alkylthiols to provide thiophene trifluoroborates in high yield with complete
regiocontrol.
P. Fricaro, L. Bialy, W. Czechtizky, M. Méndez, J. P. A. Harrity, Org. Lett.,
2018, 20, 198-200.
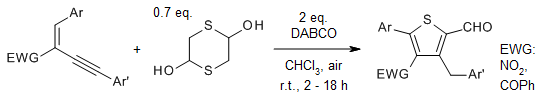
Domino synthesis of tetrasubstituted thiophenes from 1,3-enynes and
mercaptoacetaldehyde using DABCO at room temperature offers broad substrate
scope and mild reaction conditions. The reaction consists of a Michael addition,
5-exo-dig carboannulation, and oxidation sequence under air.
G. Bharathiraja, G. Sathishkannan, T. Punniyamurthy, J. Org. Chem.,
2016,
81, 2670-2674.

4-dimethylaminopyridine (DMAP) promotes an efficient and experimentally rapid
protocol for the synthesis of 2,3-dicarboalkoxy-4-aroyl/heteroaroyl/alkanoyl
thiophenes in high yields via 1-2 (C-S) and 3-4 (C-C) bond connections in only
3-5 min in DCM at room temperature. This method allows a clean and general
synthesis of previously inaccessible and synthetically demanding thiophenes.
G. C. Nandi, S. Samai, M. S. Singh, J. Org. Chem., 2011,
76, 8009-8014.

Methyl 3-aryl-2-bromo-2-chloropropanoates, which can be prepared by Meerwein
reaction from methyl 2-chloroacrylate and various arenediazonium salts under
copper(II) bromide catalysis, were used as starting materials in reactions with
substituted methanethiols for the construction of a broad range of substituted
3-hydroxythiophenes.
Y. V. Ostapiuk, M. Shehedyn, O. V. Barabash, B. Demydchuk, S. Batsyts, C.
Herzberger, A. Schmidt, Synthesis, 2022, 54,
732-740.

A highly efficient amidation reaction of heterocycles with N-fluorobenzenesulfonimide
(NFSI) presumably proceedes via C-H bond activation in the presence of cuprous
iodide as catalyst. Various α-amidated heterocycle derivatives have been
generated in good to excellent yields.
S. Wang, Z. Ni, X. Huang, J. Wang, Y. Pan, Org. Lett.,
2014,
16, 5648-5651.

N-Fluoro-N-(fluorosulfonyl)carbamate (NFC) can act as a modular
synthetic handle for one-step derivatization to amines, sulfonamides, and
sulfamides. In comparison to NFSI, NFC also offers a superior reactivity in copper-catalyzed imidations of benzene
derivatives and imidocyanation of aliphatic alkenes.
Y. Oe, R. Yoshida, A. Tanaka, A. Adachi, Y. Ishibashi, T. Okazoe, K. Aikawa,
T. Hashimoto, J. Am. Chem. Soc.,
2022, 144, 2107-2113.

A DABCO-catalyzed, one-pot, two-step, three-component reaction of
α-cyanoacetate with chalcones and elemental sulfur offers a convenient approach
to 2-aminothiophenes. This catalytic strategy offers excellent atom/step
efficiency and high degree of structural diversification by simply choosing the
suitable starting chalcones.
T. B. Nguyen, D. H. Mac, P. Retailleau, J. Org. Chem., 2021, 86,
9418-9427.

A TBAI/TBHP catalyst system enables a facile synthetis of highly functionalized
3-aminothiophenes from readily available allenes and thioamides in very good
yields under mild reaction conditions via a tandem thio-Michael addition,
oxidative annulation, and 1,2-sulfur migration pathway.
T. Han, Y. Wang, H.-L. Li, X. Luo, W.-P. Deng, J. Org. Chem.,
2018, 83, 1538-1542.

A visible-light-induced [3+2] oxidative cyclization of various alkynes with
easily available ketene dithioacetals as thiavinyl 1,3-dipoles provides
multisubstituted thiophenes in good yields under very mild metal-free conditions
in the presence of an acridine photosensitizer. This reaction tolerates a wide
range of substrates and achieves good efficiency in large-scale syntheses.
B. Zheng, X. Li, Y. Song, S. Meng, Y. Li, Q. Liu, L. Pan, Org. Lett., 2021, 23,
3453-3459.

An efficient one-pot procedure allows the synthesis of various functionalized
2-aminothiophene scaffolds catalyzed by L-proline in high yields under mild
conditions. Low catalyst loading, simple procedure, and high yields are the
important attributes of this methodology.
T. Wang, X.-G. Huang, J. Liu, B. Li, J.-J. Wu, K.-X. Chen, W.-L. Zhu, X.-Y. Xu,
B.-B. Zeng, Synlett, 2010,
1351-1354.

A simple and highly efficient protodecarboxylation of various heteroaromatic
carboxylic acids is catalyzed by Ag2CO3 and AcOH in DMSO.
This methodology enables also a selective monoprotodecarboxylation of several
aromatic dicarboxylic acids.
P. Lu, C. Sanchez, J. Cornella, I. Larrosa, Org. Lett., 2009,
11, 5710-5713.

Cross-coupling of aryl bromides with 2-thienyl, 3-thienyl, 2-pyridyl, and
3-pyridyl aluminum reagents in the presence of Pd(OAc)2 and (o-tolyl)3P
provides useful biaryl building blocks. Additionally, the catalytic system was
also suited well for the coupling reaction of benzyl halides with pyridyl
aluminum reagents to afford a series of pyridylarylmethanes.
X. Chen, L. Zhou, Y. Li, T. Xie, S. Zhou, J. Org. Chem., 2014,
79, 230-239.

Conditions for the palladium-catalyzed direct arylation of a wide range of
heterocycles with aryl bromides employ a stoichiometric ratio of both coupling
partners, as well as a substoichiometric quantity of pivalic acid, which results
in significantly faster reactions. An evaluation of the influence of the nature
of the aryl halide has also been carried out.
B. Liégault, D. Lapointe, L. Caron, A. Vlassova, K. Fagnou, J. Org. Chem., 2009,
74, 1826-1834.

Thiophene was regioselectively deprotonated by treatment with Bu3MgLi
in THF at room temperature. The lithium arylmagnesate formed was either trapped
with electrophiles or cross-coupled in a ‘one-pot’ procedure with aryl halides
under palladium catalysis.
O. Bayh, H. Awad, F. Mongin, C. Hoarau, F. Trécourt, G. Quéquiner, F. Marsais,
F. Blanco, B. Abarca, R. Ballesteros, Tetrahedron, 2005,
61, 4779-4784.

O. Bayh, H. Awad, F. Mongin, C. Hoarau, F. Trécourt, G. Quéquiner, F. Marsais,
F. Blanco, B. Abarca, R. Ballesteros, Tetrahedron, 2005,
61, 4779-4784.
