Rh-Catalyzed Enantioselective Diboration of Simple Alkenes: Reaction Development and Substrate Scope
Stéphane Trudeau, Jeremy B. Morgan, Mohanish Shrestha and James P. Morken*
*Department of Chemistry, Merkert Chemistry Center, Boston
College, Chestnut Hill, Massachusetts 02467, Email: morken bc.edu
bc.edu
S. Trudeau, J. M. Morgan, M. Shrestha, J. P. Morken, J. Org. Chem., 2005, 70, 9538-9544.
DOI: 10.1021/jo051651m

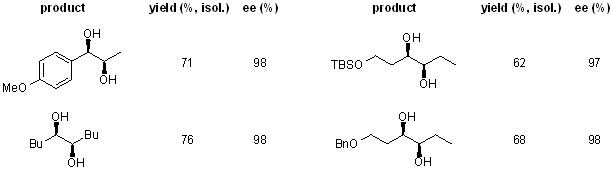
Abstract
A rhodium-catalyzed enantioselective syn addition of bis(catecholato)diboron to simple alkenes in the presence of (S)-Quinap provides enantioenriched 1,2-diols after subsequent oxidation. The substrate scope, the reaction mechanism, and competing pathways are discussed.


see article for more examples and reactions
Pt-Catalyzed Enantioselective Diboration of Terminal Alkenes with B2(pin)2
L. T. Kliman, S. N. Mlynarski, J. P. Morken, J. Am. Chem. Soc., 2009, 131, 13210-13211.
Key Words
1,2-Diols, Diboration, Hydrogen Peroxide
ID: J42-Y2005-1940
