Highly Enantioselective Conjugate Reduction of β,β-Disubstituted α,β-Unsaturated Nitriles
Daehyung Lee, Daesung Kim, Jaesook Yun*
*Department of Chemistry and Institute of Basic Science, Sungkyunkwan
University, Suwon 440-746, Korea, Email: jaesook skku.edu
skku.edu
D. Lee, D. Kim, S. Yun, Angew. Chem. Int. Ed., 2006, 45, 2785-2787.
DOI: 10.1002/anie.200600184

Abstract
A highly enantioselective reduction of α,β-unsaturated nitriles can be conducted by using a Cu(OAc)2/josiphos complex as the catalyst under hydrosilylation conditions. The reaction provides access to valuable β-aryl-substituted chiral nitriles in good yields and with excellent enantioselectivities.
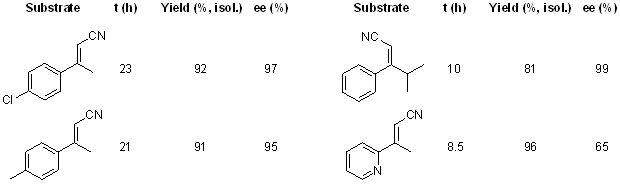
see article for more examples
Copper-Catalyzed Asymmetric Reduction of 3,3-Diarylacrylonitriles
D. Lee, Y. Yang, J. Yun, Org. Lett., 2007, 9, 2749-2751.
Key Words
Reduction of α,β-unsaturated compounds, PMHS, CuH
ID: J06-Y2006-1040
