Enantioselective Synthesis of Trifluoromethyl-Substituted Cyclopropanes
Justin R. Denton, Dinesh Sukumaran and Huw M. L. Davies*
*Department of Chemistry, Emory University, Atwood Hall 440,
Atlanta, GA 30322, Email: hmdavie emory.edu
emory.edu
J. R. Denton, D. Sukumaran, H. M. L. Davies, Org. Lett., 2007, 9, 2625-2628.
DOI: 10.1021/ol070714f

Abstract
The reaction of 1-aryl-2,2,2-trifluorodiazoethanes with alkenes provides trifluoromethyl-substituted cyclopropanes with high diastereoselectivity and enantioselectivity in the presence of an adamantylglycine-derived dirhodium complex Rh2(R-PTAD)4 as catalyst.
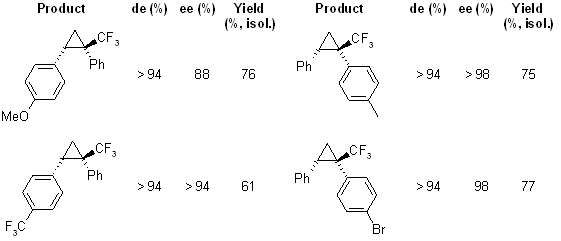
see article for more examples
H. M. L. Davies, S. J. Hedley, B. R. Bohall, J. Org. Chem., 2005, 70, 10737-10742.
Key Words
Cyclopropanes, Diazo Compounds, Manganese(IV) Oxide
ID: J54-Y2007-2120
