Reversal of regioselection in the asymmetric aminohydroxylation of cinnamates
Beata Tao, Gunther Schlingloff and K. Barry Sharpless*
*Department of Chemistry and the Skaggs Institute for
Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road,
La Jolla, California 92037, Email: sharples scripps.edu
scripps.edu
B. Tao, G. Schlingloff, K. B. Sharpless, Tetrahedron Lett., 1998, 39, 2507-2510.
DOI: 10.1021/ol000098m

see article for more reactions
Abstract
Use of cinchona ligands with an anthraquinone (AQN) core, in place of the usual phthalazine (PHAL) core, in the asymmetric aminohydroxylation of cinnamates causes dramatic reversal of the regioselection, so that phenyl serines are obtained in high enantiomeric excess. Hence, the regioselectivity is controlled by the ligand and not the substrate.
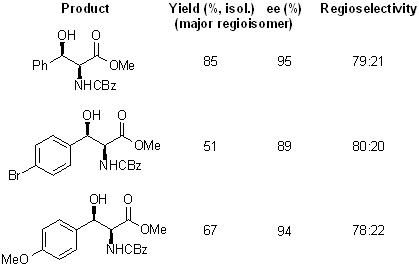 see article for more examples
see article for more examples
Primary Amides. A General Nitrogen Source for Catalytic Asymmetric Aminohydroxylation of Olefins
Z. P. Demko, M. Bartsch, K. B. Sharpless, Org. Lett., 2000, 2, 2221-2223.
Key Words
Sharpless Oxyamination, Amino Acids, Asymmetric reactions, Catalysis
ID: J72-Y1998-620
