Acryloyl Chloride: An Excellent Substrate for Cross-Metathesis. A One-Pot Sequence for the Synthesis of Substituted α,β-Unsaturated Carbonyl Derivatives
Laurent Ferrié, Samir Bouzbouz and Janine Cossy*
*Laboratoire de Chimie Organique ESPCI ParisTech, CNRS, 10
rue Vauquelin, 75231 Paris Cedex 05, France, Email: janine.cossy espci.fr
espci.fr
L. Ferrié, S. Bouzbouz, J. Cossy, Org. Lett., 2009, 11, 5446-5448.
DOI: 10.1021/ol9021386
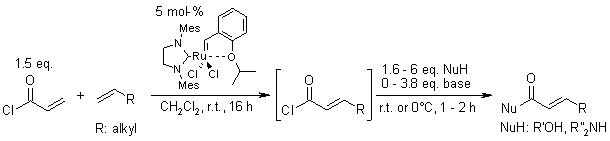
Abstract
A very simple one-pot process involving a cross-metathesis between acryloyl chloride and a terminal olefin followed by the addition of a nucleophile allows the synthesis of various functionalized α,β-unsaturated carbonyl compounds in good yield.
 see article for more examples
see article for more examples
Regioselective Cross-Metathesis Reaction Induced by Steric Hindrance
S. BouzBouz, R. Simmons, J. Cossy, Org. Lett., 2004, 6, 3465-3467.
Key Words
Cross-Metathesis, α,β-unsaturated esters and amides
ID: J54-Y2009-3420
