Hypervalent λ3-Bromane Strategy for Baeyer-Villiger Oxidation: Selective Transformation of Primary Aliphatic and Aromatic Aldehydes to Formates, Which is Missing in the Classical Baeyer-Villiger Oxidation
Masahito Ochiai*, Akira Yoshimura, Kazunori Miyamoto, Satoko Hayashi and Waro Nakanishi
*Graduate School of Pharmaceutical Sciences, University of
Tokushima, 1-78 Shomachi, Tokushima 770-8505, Japan, Email: mochiai ph.tokushima-u.ac.jp
ph.tokushima-u.ac.jp
Y. Yoshida, K. Murakami, H. Yorimitsu, K. Oshima, J. Am. Chem. Soc., 2010, 132, 9236-9239.
DOI: 10.1021/ja104330g
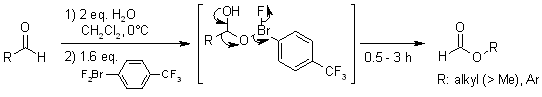
Abstract
Ligand exchange of hypervalent aryl-λ3-bromane with a carbonyl hydrate yields a new type of activated Criegee intermediate. The intermediate undergoes BV rearrangement and produces format esters for primary aliphatic as well as aromatic aldehydes via facile reductive elimination of the aryl-λ3-bromanyl group, while the attempted classical BVO produces only carboxylic acids.

see article for more examples
Key Words
Baeyer-Villiger Oxidation, Hypervalent Bromine Compounds
ID: J48-Y2010-1890
