Organocatalytic Decarboxylative Doebner-Knoevenagel Reactions between Arylaldehydes and Monoethyl Malonate Mediated by a Bifunctional Polymeric Catalyst
Jinni Lu, Patrick H. Toy*
*Department of Chemistry, The University of Hong Kong,
Pokfulam Road, Hong Kong, P. R. of China, Email: phtoy hku.hk
hku.hk
J. Lu, P. H. Toy, Synlett, 2011, 1723-1726.
DOI: 10.1055/s-0030-1260808
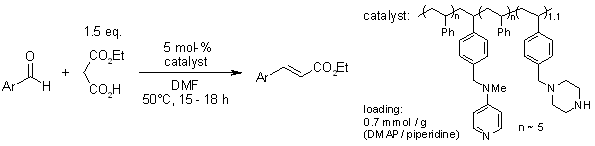
Abstract
A bifunctional polystyrene bearing both DMAP and piperidine groups is an effective organocatalyst for decarboxylative Doebner-Knoevenagel reactions of arylaldehydes and monoethyl malonate to give E-cinnamates in high yields. A synergistic effect obtained by co-locating the two different catalytic amine groups on the same polymer backbone has been detected.
 see article for more examples
see article for more examples
Key Words
Doebner-Knoevenagel reaction, organocatalysis, polystyrene, DMAP, cinnamates
ID: J60-Y2011-2030
