DABCO-Bis(sulfur dioxide), DABSO, as a Convenient Source of Sulfur Dioxide for Organic Synthesis: Utility in Sulfonamide and Sulfamide Preparation
Holly Woolven, Carlos González-Rodríguez, Isabel Marco, Amber L. Thompson and Michael C. Willis*
*Department of Chemistry, University of Oxford, Chemistry
Research Laboratory, Mansfield Road, Oxford, OX1 3TA, U.K.,
Email: michael.willis chem.ox.ac.uk
chem.ox.ac.uk
H. Woolven, C. Gonzáles-Rodríguez, I. Marco, A. L. Thompson, M. C. Willis, Org. Lett., 2011, 13, 4876-4878.
DOI: 10.1021/ol201957n

see article for more reactions
Abstract
The bench-stable colorless solid charge-transfer complex generated from the combination of DABCO and sulfur dioxide, DABSO, can replace gaseous sulfur dioxide in organic synthesis. Reactions with Grignard reagents form sulfinates, which can then be converted in situ to sulfonamides. Alternatively, reaction with anilines and iodine leads to the formation of a series of sulfamides.
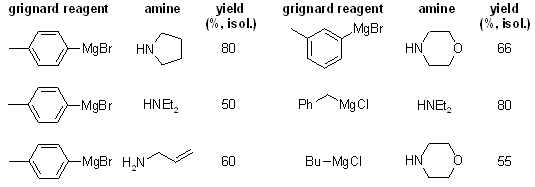
see article for more examples
T. Q. Davies, M. J. Tilby, D. Skolc, A. Hall, M. C. Willis, Org. Lett., 2020, 22, 9495-9499.
M. Ding, Z.-X. Zhang, T. Q. Davies, M. C. Willis, Org. Lett., 2022, 24, 1711-1715.
Sulfinamide Synthesis Using Organometallic Reagents, DABSO, and Amines
P. K. T. Lo, G. A. Oliver, M. C. Willis, J. Org. Chem., 2020, 85, 5753-5760.
One-Pot Sulfonamide Synthesis Exploiting the Palladium-Catalyzed Sulfination of Aryl Iodides
E. F. Flegeau, J. M. Harrison, M. C. Willis, Synlett, 2016, 27, 101-105.
Key Words
ID: J54-Y2011-2700
