i-Pr2NMgCl·LiCl Enables the Synthesis of Ketones by Direct Addition of Grignard Reagents to Carboxylate Anions
Kilian Colas, A. Catarina V. D. dos Santos and Abraham Mendoza*
*Department of Organic Chemistry, Stockholm University, Arrhenius Laboratory, 106 91 Stockholm, Sweden,
Email: abraham.mendoza su.se
su.se
K. Colas, A. C. V. D. dos Santos, A. Mendoza, Org. Lett., 2019, 21, 7851-7856.
DOI: 10.1021/acs.orglett.9b02899
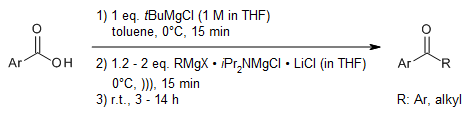
see article for more reactions
Abstract
A magnesium amide additive activates and controls the addition of mild Grignard reagents to carboxylate anions and therefore avoids the use of organolithium reagents or activated acyl sources that need to be independently prepared. This strategy enables the modular synthesis of ketones from CO2 and the preparation of isotopically labeled pharmaceutical building blocks in a single operation.

see article for more examples
Key Words
acylation (ketones, aryl ketones), Grignard reaction
ID: J54-Y2019
