Access to Substituted Thiophenes through Xanthate-Mediated Vinyl C(sp2)-Br Bond Cleavage and Heterocyclization of Bromoenynes
Guoling Huang, Jian Li, Jianrong Li, Jiaming Li, Minghua Sun, Peng Zhou, Lu Chen, Yubing Huang, Shaohua Jiang* and Yibiao Li*
*School of Biotechnology and Health Sciences, Wuyi University, Jiangmen, Guangdong Province 529090, China,
Email: wyuchemjsh 126.com, Leeyib268
126.com, Leeyib268 126.com
126.com
G. Huang, J. Li, J. Li, J. Li, M. Sun, P. Zhou, L. Chen, Y. Huang, S. Jiang, Y. Li, J. Org. Chem., 2020, 85, 13037-13049.
DOI: 10.1021/acs.joc.0c01733
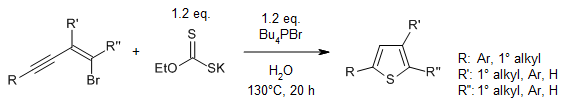
see article for more reactions
Abstract
In an environmentally sustainable strategy for the chemoselective heterocyclization of bromoenynes through a sulfuration/cyclization process, inexpensive and safe EtOCS2K is used as a thiol surrogate and tetrabutylphosphonium bromide and H2O as a mixed solvent. This transition-metal-free reaction provides a range of substituted thiophenes in good yields.

see article for more examples
Synthesis of Substituted Thiophenes through Dehydration and Heterocyclization of Alkynols
J. Li, Y. Liu, Z. Chen, J. Li, j. Li, X. Ji, L. Chen, Y. Huang, Q. Liu, Y. Li, J. Org. Chem., 2022, 87, 3555-3566.
Key Words
ID: J42-Y2020
