Highly Efficient Stereoconservative Amidation and Deamidation of α-Amino Acids
Deepak M. Shendage, Roland Fröhlich and Günter Haufe*
*Organisch-Chemisches Institut, Universität Münster, Corrensstrasse 40,
D-48149 Münster, Germany, Email: haufe uni-muenster.de
uni-muenster.de
D. M. Shendage, R. Froehlich, G. Haufe, Org. Lett., 2004, 6, 3675-3678.
DOI: 10.1021/ol048771l
 Amidation
Amidation
 Deamidation
Deamidation
Abstract
A stereoconservative protection and deprotection method of amino and carboxyl groups includes the generation of N-Phthaloyl N'-alkyl secondary amides from N-phthaloyl amino acids by using a mixed anhydride method. These secondary amides have been transformed by thermal rearrangement of the intermediate nitrosoamides to esters with retention of configuration and excellent yields.
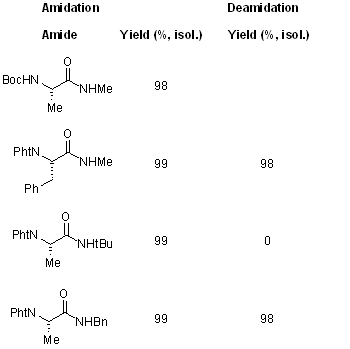
see article for more examples
N-Protection of Amino Acids


see article for more examples
N-Deprotection of Amides


see article for more examples
Key Words
amidation, amides, deamidation, esters, tert-butyl carbamates
ID: J54-Y2004-830
