N-Chlorosuccinimide (NCS)
N-Chlorosuccinimide (NCS) is a chlorinating and oxidizing agent that is used as source for chlorine in radical reactions and various electrophilic additions.
Recent Literature

N-Halosuccinimides are efficiently activated in
trifluoromethanesulfonic acid and BF3-H2O, allowing
the halogenations of deactivated aromatics. BF3-H2O is
more economic, easy to prepare, nonoxidizing, and offers sufficiently high
acidity.
G. K. S. Prakash, T. Mathew, D. Hoole, P. M. Esteves, Q. Wang, G. Rasul, G.
A. Olah, J. Am. Chem. Soc.,
2004,
126, 15770-15776.

A mild, efficient, Cu(I)-catalyzed method for the synthesis of aryl chlorides
from arylboronic acids is particularly useful for the conversion of
electron-deficient arylboronic acids to aryl chlorides, a transformation that is
inefficient in the absence of Cu catalysis.
H. Wu, J. Hynes, Jr., Org. Lett., 2010,
12, 1192-1195.

Selective oxyhalogenations of alkynes were achieved in water under very mild
conditions in the presence of inexpensive halogenating reagents, such as N-bromosuccinimide
and N-chlorosuccinimde, and FI-750-M as amphiphile. No halogenation at
the aromatic rings was detected. Reaction medium and catalyst can be recycled.
L. Finck, J. Brals, B. Pavuluri, F. Gallou, S. Handa, J. Org. Chem., 2018, 83,
7366-7372.

The Lewis base Trip-SMe (Trip = triptycenyl) catalyzes an electrophilic
halogenation of unactivated aromatic compounds using N-halosuccinimides (NXS) at
ambient temperature in the presence of AgSBF6 as source of a
non-coordinating anion. The π system of the triptycenyl functionality exerts a
crucial role for the enhancement of electrophilicity.
Y. Nishii, M. Ikeda, Y. Hayashi, S. Kawauchi, M. Miura, J. Am. Chem. Soc.,
2020, 142, 1621-1629.

A highly para-selective halogenation of arenes bearing electron-donating
coordinating groups in the presence of a dimidazolium salt rpovides p-haloarenes
in good yields. A plausible mechanism for the catalytic reaction is proposed.
J. Chen, X. Xiong, Z. Chen, J. Huang,
Synlett, 2015, 26, 2831-2834.

Using cyano as the directing group, a palladium-catalyzed ortho-halogenation
(I, Br, Cl) reaction gave good to excellent yields. The method is compatible to
arylnitriles with either electron-withdrawing or electron-donating groups. The
present method was successfully applied to the synthesis of the precursors of
paucifloral F and isopaucifloral F.
B. Du, X. Jiang, P. Sun, J. Org. Chem., 2013,
78, 2786-2791.

Silver carbonate or nickel(II) chloride catalyzes an ortho-C-H bond halogenation of anilides and
N-aryl carbamates with N-halosuccinimides to provide 2-haloanilides and carbamates, which may serve as starting
materials for the synthesis of pharmaceutically and biologically active
compounds.
E. Kianmehr, H. Afaridoun, Synthesis, 2021, 53,
1513-1523.

A mild palladium-catalyzed, regioselective chlorination, bromination, and
iodination of arene C-H bonds using N-halosuccinimides as oxidants is
described. These transformations can provide products that are complementary
to those obtained via conventional electrophilic aromatic substitution
reactions.
D. Kalyani, A. R. Dick, W. Q. Anani, M. S. Sanford, Org. Lett.,
2006,
8, 2523-2526.

The combination of N-chlorosuccinimide as safe chlorine source with
Acr+-Mes as the photocatalyst achieves a benzylic C-H bond
chlorination under visible light irradiation. This mild and scalable
chlorination method worked effectively for electron-deficient substrates.
Furthermore, benzylic chlorides could be converted to benzylic ethers smoothly
in one-pot by adding sodium methoxide.
M. Xiang, C. Zhou, X.-L. Yang, B. Chen, C.-H. Tung, L.-Z. Wu, J. Org. Chem., 2020, 85,
9080-9087.

Substoichiometric amounts of thiourea additives mediate the halogenation of
alcohols under mild conditions. In the the absence of thiourea, oxidation of the
alcohol is observed, whereas the substrate can be recovered when excess thiourea is
used. Both bromination and chlorination were highly efficient for primary,
secondary, tertiary, and benzyl alcohols and tolerate a broad range of
functional groups.
A. R. Mohite, R. S. Phatake, P. Dubey, M. Agbaria, A. I. Shames, N. G.
Lemcoff, O. Reany, J. Org. Chem., 2020, 85,
12901-12911.

Using triethylamine as catalyst in Hunsdiecker reactions with N-halosuccinimides
as Br+ or I+ source, cinnamic acids, and propiolic acids are
converted to the corresponding α-halostyrenes and 1-halo-1-alkynes in good isolated yields within 1-5 min.
J. Prakash, S. Roy, J. Org. Chem.,
2002, 67, 7861-7864.

Using an efficient visible-light photocatalysis-based method, a mixture of an
aldehyde, tert-butyl hydrogen peroxide, and N-chlorosuccinimide
afforded an acid chloride in the presence of Ru(bpy)3Cl2
as photocatalyst. A subsequent reaction with an amine provided the corresponding
amide.
N. Iqbal, E. J. Cho, J. Org. Chem.,
2016,
81, 1905-1911.

Stable zwitterionic compounds catalyze a synthesis of α,α-dihalo-N-arylacetamides
from β-oxo amides and N-halosuccinimides as the halogen sources. The
corresponding α,α-dihalo-N-arylacetamides were obtained in very good
yields under mild conditions without strong base or acid.
Z. Ke, Y.-P. Lam, K.-S. Chan, Y.-Y. Yeung,
Org. Lett., 2020, 22, 7353-73572.

A photochemical Wolff rearrangement, trapping of the generated ketene with a
chiral Lewis base catalyst, subsequent enantioselective α-chlorination, and a
final nucleophilic displacement of the bound catalyst provides chiral
α-chlorinated carboxylic acid esters. The obtained products were successfully
utilized for stereospecific nucleophilic displacement reactions with N-
and S-nucleophiles.
D. Weinzierl, M. Piringer, P. Zebrowski, L. Stockhammer, M. Waser, Org. Lett., 2023, 25,
3126-3130.

A facile and efficient method for preparing N-tosylhydrazonyl chlorides
in good yields is general for a variety of substrates
and displays high functional group compatibility.
Q. Zhang, S. Zhang, M. Tang, J. Org. Chem., 2022, 87,
6393-6396.

An operationally simple oxidation-cyanation enables the synthesis of
cyanamides using inexpensive and commercially available N-chlorosuccinimide
and Zn(CN)2 as reagents. The method avoids the direct handling of
toxic cyanogen halides and is amenable for the cyanation of various primary and
secondary amines and aniline derivatives.
N. Kuhl, S. Raval, R. D. Cohen, Org. Lett., 2019, 21,
1268-1272.

Treatment of anilines with N-chlorosuccinimide and
1,8-diazabicyclo[5.4.0]undec-7-ene enables a convenient one-step procedure for
the synthesis of symmetrical azobenzenes in good yields in minutes.
A. A. John, Q. Lin, J. Org. Chem.,
2017, 82, 9873-9876.

A facile synthesis of hydrazides from N-tosylhydrazones under
metal-free conditions was suitable for different substrates and tolerated
various substituents. In a subsequent step, a series of indazolones could be
prepared from corresponding o-bromobenzohydrazides under mild conditions.
S. Zhang, Q. Zhang, M. Tang, J. Org. Chem., 2022, 87,
3845-3850.

The use of nBuLi as nucleophilic reagent to attack a boron atom in
gem-diborylalkanes to form a tetracoordinate boron species, followed by
reaction with readily accessible electrophilic halogen reagents (NCS and NBS)
provides α-chloroboronates and α-bromoboronates. The reaction is
transition-metal-free and features a broad substrate scope.
S. Liao, J. Liang, C. Li, N. Chen, K. Yang, J. Chen, Q. Song, Org. Lett., 2023, 25,
2938-2933.

An intramolecular chloroamination of allenes with N-chlorosuccinimide
proceeds under mild conditions in the presence of a 1,10-phenanthroline-ligated
cationic silver complex and 2,6-lutidine as a base. The reaction tolerates
various functional groups. The chloroamination products are useful synthetic
intermediates and can be easily transformed into functionalized 3-pyrroline and
pyrrole derivatives.
M. Sai, S. Matsubara, Org. Lett., 2011,
13, 4676-4679.

The use of secondary amines and NXS (X = Cl, Br, I) enables an unprecedented
copper-catalyzed oxidative aminohalogenation of electron-deficient maleimides.
This simple and green multicomponent reaction offers late-stage modification of
drug molecules and in situ formation of N-iodoamines for efficient alkene
aminoiodination.
X. Zhou, Y. Yao, C. Wang, Y. Xu, W. Zhang, Y. Ma, G. Wu, Org. Lett., 2021, 23,
3669-3673.

A Pd-catalyzed enantioselective aminochlorination of alkenes via a 6-endo
cyclization provides convenient access to a wide array of structurally diverse
3-chloropiperidines in good yields with excellent enantioselectivities. Notably,
both the sterically bulky chiral pyridinyl-oxazoline (Pyox) ligand and an
electrophilic chlorination reagent (NCS) are crucial to the successful reaction.
Y. Zhang, Y. He, T. Liu, W. Guo, Org. Lett., 2023, 25,
2680-2684.
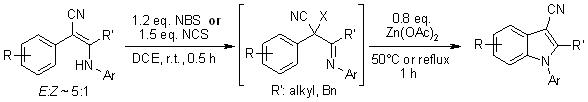
Various 2-aryl-3-arylamino-2-alkenenitriles give N-arylindole-3-carbonitriles
in a one-pot manner through NBS- or NCS-mediated halogenation followed by
Zn(OAc)2-catalyzed intramolecular cyclization. The process involves
the formation of arylnitrenium ion intermediates, which undergo an electrophilic
aromatic substitution to give the cyclized N-arylindoles.
Q. Yan, J. Luo, D. Zhang-Negrerie, H. Li, X. Qi, K. Zhao, J. Org. Chem., 2011,
76, 8690-8697.

A one-pot, cascade reaction sequence of α-azido acrylates and aromatic oximes
provides an efficient, straightforward and metal-free synthesis of
3,4,5-trisubstituted isoxazoles under mild reaction conditions via a 1,3-dipolar
cycloaddition.
M. Hu, X. He, Z. Niu, Z. Yan, F. Zhou, Y. Shang, Synthesis, 2014, 46,
510-514.

Various primary alcohols were smoothly transformed into 3-substitued
isoxazoles in good yields in one pot by successive treatment with PhI(OAc)2
in the presence of TEMPO, NH2OH, and then NCS, followed by reaction
with alkynes in the presence of Et3N. The use of PhNHNH2
instead of NH2OH and decyl methyl sulfide as additive in a later step enabled the synthesis of pyrazoles.
E. Kobayashi, H. Togo, Synthesis, 2019, 51,
3723-3735.

Various primary alcohols were smoothly transformed into 3-substitued
isoxazoles in good yields in one pot by successive treatment with PhI(OAc)2
in the presence of TEMPO, NH2OH, and then NCS, followed by reaction
with alkynes in the presence of Et3N. The use of PhNHNH2
instead of NH2OH and decyl methyl sulfide as additive in a later step enabled the synthesis of pyrazoles.
E. Kobayashi, H. Togo, Synthesis, 2019, 51,
3723-3735.

A divergent and regioselective synthesis of either 3-substituted benzisoxazoles
or 2-substituted benzoxazoles from readily accessible ortho-hydroxyaryl
N-H ketimines proceeds in two distinct pathways through a common N-Cl imine
intermediate: (a) N-O bond formation to form benzisoxazole under anhydrous
conditions and (b) NaOCl mediated Beckmann-type rearrangement to form
benzoxazole, respectively.
C.-y Chen, T. Andreani, H. Li, Org. Lett., 2011,
13, 6300-6303.

A chlorinative cyclization of aryl alkynoates in the presence of N-chlorosuccinimide
(NCS) and Mes-Acr-MeClO4 as photocatalyst provides 3-chlorocoumarins
under visible-light irradiation. The radical initiated reaction proceeds via Cl-
addition, 5-exo-trig spirocyclization and subsequent 1,2-ester migration.
M. Pramanik, A. Mathuri, S. Sau, M. Das, P. Mal, Org. Lett., 2021, 23,
8088-8092.

A fluorous (S)-pyrrolidine-thiourea bifunctional organocatalyst shows
good activity and enantioselectivity for direct α-chlorination of aldehydes
using N-chlorosuccinimide (NCS) as the chlorine source. The catalyst can
be recovered from the reaction mixture by fluorous solid-phase extraction with
excellent purity for direct reuse.
L. Wang, C. Cai, D. P. Curran, W. Zhang, Synlett, 2010, 433-436.

A direct organocatalytic enantioselective α-chlorination of aldehydes
proceeds for a series of different aldehydes with NCS as the chlorine source
using easily available catalysts such as L-proline amide and (2R,5R)-diphenylpyrrolidine.
The α-chloro aldehydes are obtained in very good yield and high
enantioselectivity.
N. Halland, A. Braunton, S. Bachmann, M. Marigo, K. A. Jorgensen, J. Am. Chem. Soc.,
2004,
126, 4790-4791.

A Rh(III)-catalyzed cascade arylation and chlorination of α-diazocarbonyl
compounds with arylboronic acids and N-chlorosuccinimide exhibits
excellent functional group tolerance on the organoboron and the diazo reagents.
Functionalized α-aryl-α-chlorocarbonyl compounds were obtained in good yields.
F.-N. Ng, Y.-F. Lau, Z. Zhou, W.-Y. Yu, Org. Lett.,
2015,
17, 1676-1679.

The use of diphenyl selenide as a Lewis base catalyst enables a mild
chloroamidation of a wide rand of olefinic substrates including starting
materials with acid labile functional groups.
D. W. Tay, I. T. Tsoi, J. C. Er, G. Y. C. Leung, Y.-Y. Yeung, Org. Lett., 2013,
15, 1310-1313.

Structurally diverse sulfonyl chlorides were synthesized via N-chlorosuccinimide
chlorosulfonation in good yields from S-alkylisothiourea salts, which can
be easily prepared from readily accessible alkyl halides or mesylates and
inexpensive thiourea. In large-scale syntheses, the water-soluble byproduct
succinimide can be conveniently converted into the starting reagent N-chlorosuccinimide
with sodium hypochlorite.
Z. Yang, J. Xu, Synthesis, 2013, 45,
1675-1682.

A smooth oxidation of several thiol derivatives by a combination of N-chlorosuccinimide
and dilute hydrochloric acid afforded the corresponding sulfonyl chlorides in
good yield.
A. Nishiguchi, K. Maeda, S. Miki,
Synthesis, 2006, 4131-4134.

In situ preparation of sulfonyl chlorides from thiols by oxidation with N-chlorosuccinimide
(NCS), tetrabutylammonium chloride, and water followed by reaction with amine or
sodium azide in the same reaction vessel enables a convenient synthesis of
sulfonamides and sulfonyl azides.
H. Veisi, R. Ghorbani-Vaghei, S. Hemmati, J. Mahmoodi, Synlett, 2011,
2315-2320.

A method for the synthesis of N-aroylated sulfoximines involves a
manganese oxide promoted C-H activation of methyl arenes to form an aroyl
intermediate which then reacts readily with N-chlorosulfoximines to form
a series of valuable aroyl sulfoximine derivatives in high yields.
D. L. Priebbenow, C. Bolm, Org. Lett., 2014,
16, 1650-1652.

A one-pot, two-step mechanochemical synthesis of sulfonimidamides requires
neither a solvent nor inert conditions. In a mixer mill, sulfinamides are
rapidly converted to sulfonimidoyl chlorides by oxidative chlorination with N-chlorosuccinimide
(NCS). Subsequent substitutions with amines provides a wide range of diversely
substituted sulfonimidamides.
S. Terhorst, T. Jansen, T. Langletz, C. Bolm, Org. Lett.,
2022, 24, 4109-4113.
