TEMPO, 2,2,6,6-Tetramethylpiperidinyloxy
2,2,6,6-Tetramethylpiperidinyloxy is a stable nitroxyl radical, which serves in oxidations as catalyst. By substitution in position 4 (4-hydroxy-TEMPO, 4-acetamido-TEMPO) its reactivity can be steered.

For example, processes with oxygen and 5 mol-% TEMPO permit environmentally benign reactions as alternatives to some oxidations, where so far e.g. chrome reagents were used.

Proposed mechanism for the oxidation of primary alcohols under basic
conditions
M. Angelin, M. Hermansson, H. Dong, O. Ramström, Eur. J. Org. Chem., 2006,
4323-4326.
2-Azaadamantane N-Oxyl (AZADO), 9-Azabicyclo[3.3.1]nonane N-Oxyl (ABNO), 9-Azanoradamantane N-oxyl (Nor-AZADO) form a less hindered class of nitroxyl radicals and exhibit an enhanced reactivity compared with TEMPO.
Recent Literature
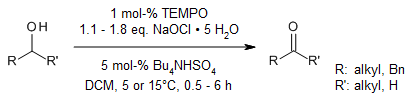
Sodium hypochlorite pentahydrate crystals with very low NaOH and NaCl contents
oxidize primary and secondary alcohols to the corresponding aldehydes and
ketones in the presence of TEMPO/Bu4NHSO4 without pH
adjustment. This new oxidation method is also applicable to sterically hindered
secondary alcohols.
T. Okada, T. Asawa, Y. Sugiyama, M. Kirihara, T. Iwai, Y. Kimura, Synlett, 2014, 25,
596-598.

Oxidation of primary and secondary alcohols, using catalytic amounts of TEMPO
and tetra-n-butylammonium bromide in combination with periodic acid and
wet alumina in dichloromethane is compatible with a broad range of functional
groups and acid-sensitive protecting groups. The system also enables a
chemoselective oxidation of secondary alcohols in the presence of primary
alcohols.
M. Attoui, J.-M. Vatèle,
Synlett, 2014, 25, 2923-2927.

A convenient method enables the preparation of a silica gel supported
TEMPO catalyst. The catalyst prepared from [4-hydroxy-TEMPO + NaCl]/SiO2
was used for an aerobic oxidation of alcohols to carbonyls under mild reaction
conditions in the presence of Fe(NO3)3 • 9 H2O.
Alcohols were converted to the corresponding carbonyls in good to excellent
yields. After a simple filtration, the catalyst can be reused at least six times.
N. Tamura, T. Aoyama, T. Takido, M. Kodomari, Synlett, 2012, 23,
1397-1407.

A rapid oxidation of primary and secondary alcohols using catalytic amounts of
TEMPO and Yb(OTf)3 in combination with a stoichiometric amount of
iodosylbenzene afforded carbonyl compounds in excellent yields without
over-oxidation. Oxidation of primary alcohols in the presence of secondary
alcohols proceeded with good selectivity.
J.-M. Vatèle, Synlett, 2006,
2055-2058.

An aerobic oxidation of primary and secondary alcohols to aldehydes and
ketones using TEMPO-CuCl as catalyst in the ionic liquid [bmin][PF6]
has been developed. The system needs no bubbling of O2 due to
its good solubility in the ionic liquid. The resulting aldehydes (with
no traces of carboxylic acids) and ketones can be extracted with organic
solvents. The ionic liquid can be reused after washing with water and
drying under high vacuum (8 runs for the oxidation of benzyl alcohol:
yields of 72%, 70, 68, 70, 65, 64, 62, and 60).
I. A. Ansari, R. Gree, Org. Lett., 2001, 1507-1509.

A highly efficient 2,2,6,6-tetramethylpiperidin-1-yloxy (TEMPO) catalyzed
reaction using recyclable 1-chloro-1,2-benziodoxol-3(1H)-one as the
terminal oxidant allows the conversion of various alcohols to their
corresponding carbonyl compounds in high to excellent yields at room temperature
in ethyl acetate, which is an environmentally friendly organic solvent.
X.-Q. Li, C. Zhang, Synthesis, 2009,
1163-1169.

The combination of TEMPO and CAN can be used for the aerobic oxidation
of benzylic and allylic alcohols into their corresponding carbonyl
compounds. However, steric hindrance has been observed to impede the
reaction with some substituted allylic systems. The present method is
superior to others currently available due to its relatively short
reaction times and excellent yields.
S. S. Kim, H. C. Jung, Synthesis,
2003, 2135-2137.

The reaction of KBrO3 and NH2OH • HCl in situ generates NOx
and Br anion, which allows in the presence of 2,2,6,6-tetramethylpiperidine-N-oxide
(TEMPO) an activation of dioxygen to oxidize various benzylic alcohols
quantitatively to their corresponding carbonyl compounds under mild conditions.
G. Yang, W. Wang, W. Zhu, C. An, X. Gao, M. Song, Synlett, 2010,
437-440.

TEMPO-derived reagents tagged with multiple perfluoroalkyl chains and
triazole moieties promote the oxidation of alcohols to aldehydes in organic
solvent/water mixtures with reaction rates comparable to homogeneous TEMPO
reagents, but can be easily recovered by liquid/emulsion filtration.
A. Gheorghe, T. Chinnusamy, E. Cuevas-Yañez, P. Hilgers, O. Reiser, Org. Lett.,
2008,
10, 4171-4174.

A. Gheorghe, T. Chinnusamy, E. Cuevas-Yañez, P. Hilgers, O. Reiser, Org. Lett.,
2008,
10, 4171-4174.

Silica-supported TEMPO is easily obtained in a one-step reductive amination
procedure starting from a commercially available aminopropyl-functionalized
silica. This supported catalyst mediates the Anelli oxidation of various
alcohols. The recyclability and stability of the applied silica-supported TEMPO
have been studied.
T. Fey, H. Fischer, S. Bachmann, K. Albert, C. Bolm, J. Org. Chem., 2001,
66, 8154-8159.

A four-component system consisting of acetamido-TEMPO/Cu(ClO4)2/TMDP/DABCO
in DMSO allows an efficient room-temperature aerobic alcohol oxidation
of various alcohols into their corresponding aldehydes or ketones in
good to excellent yields. The catalytic system can be recycled.
N. Jiang, A. J. Ragauskas, J. Org. Chem., 2006,
71, 7087-7090.

The system Cu(ClO4)2/acetamido-TEMPO/DMAP catalyses
the room-temperature aerobic oxidation of primary alcohols to aldehydes in
the ionic liquid [bmpy]PF6. The catalysts can be recycled and
reused.
N. Jiang, A. J. Ragauskas, Org. Lett., 2005,
7, 3689-3692.

An efficient oxidation of primary alcohols to the corresponding aldehydes can be
carried out at room temperature in DCM, using trichloroisocyanuric acid in the
presence of catalytic TEMPO: aliphatic, benzylic, and allylic alcohols, and
β-amino alcohols are rapidly oxidized without no overoxidation to carboxylic
acids. The slow oxidation of secondary carbinols makes the reaction highly
chemoselective.
L. De Luca, G. Giacomelli, A. Porcheddu, Org. Lett., 2001,
3, 3041-3043.

A highly efficient and mild procedure for the oxidation of different types of
alcohols uses TEMPO as catalyst, iodobenzene dichloride as stoichiometric
oxidant, and pyridine as base. Oxidation of 1,2-diols gives α-hydroxy ketones or
α-diketones depending on the amount of oxidant used. High yielding procedures
for the preparation of iodoarene dichlorides have been developed.
X.-F. Zhao, C. Zhang, Synthesis, 2007, 551-557.

A stable nitroxyl radical class of catalysts, 2-azaadamantane N-oxyl (AZADO) and
1-Me-AZADO, exhibit superior catalytic proficiency to TEMPO, converting various
sterically hindered alcohols to the corresponding carbonyl compounds in
excellent yields.
M. Shibuya, M. Tomizawa, I. Suzuki, Y. Iwabuchi, J. Am. Chem. Soc., 2006,
128, 8412-8413.

A combination of FeCl3, L-valine and TEMPO oxidizes a wide range of
primary/secondary benzyl, allylic, and heterocyclic alcohols to aldehydes and
ketones with good to excellent isolated yields in the presence of oxygen.
G. Zhang, S. Li, J. Lei, G. Zhang, X. Xie, C. Ding, R. Liu,
Synlett, 2016, 27, 956-960.

An iron-catalyzed α,β-dehydrogenation of carbonyl compounds converts a broad
range of aldehydes, ketones, lactones, lactams, amines, and alcohols to their
α,β-unsaturated counterparts in a simple one-step reaction with high yields.
X.-W. Zhang, G.-Q. Jiang, S.-H. Lei, X.-H. Shan, J.-P. Qu, Y.-B. Kang, Org. Lett., 2021, 23,
1611-1615.

An eco-friendly and mild aerobic oxidation of allylic alcohols using Fe(NO3)3·9H2O/TEMPO/NaCl
as catalysts under atmospheric pressure of oxygen at room temperature provides a
convenient pathway to the synthesis of stereodefined α,β-unsaturated enals or
enones with the retention of the double-bond configuration.
J. Liu, S. Ma, Org. Lett., 2013,
15, 5150-5153.

A method for generating (E)-α,β-unsaturated aldehydes from Z- or
E-allylic alcohols involves a Cu-catalyzed oxidation followed by an
organocatalytic Z/E-isomerization with N,N-dimethylaminopyridine
(DMAP).
D. Könning, W. Hiller, M. Christmann, Org. Lett., 2012,
14, 5258-5261.

X.-F. Zhao, C. Zhang, Synthesis, 2007,
551-557.

The use of NaClO/TEMPO/Co(OAc)2 enabled a benzylic oxidation of alkyl
arenes to yield various aromatic aldehydes and ketones in very good yields. The
reaction reactivity, selectivity, and scope of the reaction were investigated.
C. Jin, L. Zhang, W. Su, Synlett, 2011,
1435-1438.

A one-pot two-step sequence involving an oxidation/imine-iminium formation/reduction
allowed the N-alkylation of amines by alcohols. Optically active alcohols
and amines can be converted without any epimerization.
C. Guérin, V. Bellosta, G. Guillamot, J. Cossy, Org. Lett., 2011,
13, 3478-3481.

A sequential one-pot synthesis for the oxidation of primary and secondary
tert-butyldimethylsilyl (TBDMS) ethers, using the presence of PhIO or
PhI(OAc)2 and catalytic amounts of metal triflates and TEMPO in THF
or acetonitrile tolerates acid-sensitive protecting groups and leaves tert-butyldiphenylsilyl
ethers and phenolic TBDMS groups untouched.
B. Barnych, J.-M. Vatèle, Synlett, 2011,
2048-2052.

Oxidation from alcohols to carboxylic acids are often conducted using at least a
stoichiometric amount of an expensive and toxic oxidant. An efficient and
practical sustainable oxidation technology of alcohols using pure O2
or even air as the oxidant in the presence of a catalytic amount each of Fe(NO3)3·9H2O/TEMPO/MCl
provides a series of carboxylic acids in high yields at room temperature.
X. Jiang, J. Zhang, S. Ma, J. Am. Chem. Soc., 2016,
138, 8344-8347.

Catalytic amounts of TEMPO and NaOCl enable a chemoselective oxidation of
1,2-diols to in the presence of NaClO2 as terminal oxidant. The use
of a two-phase condition suppresses the concomitant oxidative cleavage. The
observed selectivity seems to be derived from the precise solubility control of
diols and hydroxy acids as well as the charge transfer complex TEMPO-ClO2,
which dissolves into the organic layer.
K. Furukawa, M. Shibuya, Y. Yamamoto, Org. Lett.,
2015,
17, 2282-2285.

A copper(II)-promoted denitrogenation/oxidation reaction of α-azido ketones
and TEMPO as an oxidant provides primary α-ketoamides. α-Azido ketones were
denitrogenated in situ to form an imino ketone intermediate, which underwent a
radical addition process and radical migration to form α-ketoamides.
A.-J. Wang, C.-Y. She, Y.-D. Zhang, L.-H. Zhao, W.-M. Shu, W.-C. Yu, J. Org. Chem., 2022, 87,
16099-16105.

In a direct conversion of primary and secondary alcohols into the corresponding
α-chloro aldehydes and α-chloro ketones, trichloroisocyanuric acid serves both
as stoichiometric oxidant and α-halogenating reagent. For primary alcohols,
TEMPO has to be added as an oxidation catalyst, and for the transformation of
secondary alcohols MeOH as an additive is essential to
promote chlorination of the intermediary ketones.
Y. Jing, C. G. Daniliuc, A. Studer, Org. Lett.,
2014,
16, 4932-4935.

In a direct conversion of primary and secondary alcohols into the corresponding
α-chloro aldehydes and α-chloro ketones, trichloroisocyanuric acid serves both
as stoichiometric oxidant and α-halogenating reagent. For primary alcohols,
TEMPO has to be added as an oxidation catalyst, and for the transformation of
secondary alcohols MeOH as an additive is essential to
promote chlorination of the intermediary ketones.
Y. Jing, C. G. Daniliuc, A. Studer, Org. Lett.,
2014,
16, 4932-4935.

An oxidation/imine-iminium formation/reduction cascade using
TEMPO-BAIB-HEH-Brønsted acid catalysis in DMPU as solvent enables a mild and
atom-economical nonepimerizing chemo- and enantioselective N-alkylating
procedure of amines with alcohols.
I. A. Khan, A. K. Saxena, J. Org. Chem., 2013,
78, 11656-11669.

A highly regio- and enantioselective hydroxyamination of aldehydes with in situ
generated nitrosocarbonyl compounds from hydroxamic acid derivatives was
realized by combining TEMPO and BPO as oxidants in the presence of a
binaphthyl-modified amine catalyst.
T. Kano, F. Shirozu, K. Maruoka, J. Am. Chem. Soc., 2013,
135, 17735-17738.

Various alcohols were efficiently converted into the
corresponding nitriles at room temperature by treatment with
tert-butyl hypochlorite, diiodine, or 1,3-diiodo-5,5-dimethylhydantoin (DIH) in the presence of TEMPO, followed by
treatment with diiodine and aqueous ammonia. The nitriles were obtained in good
yields and high purities by a simple extraction of the reaction mixture with
chloroform and subsequent removal of the solvent.
H. Shimojo, K. Moriyama, H. Togo, Synthesis, 2013, 45,
2155-2156.

In the presence of a catalytic amount of 4-AcNH-TEMPO, NaNO2, and HNO3,
benzaldehydes underwent condensation with NH4OAc and a subsequent
aerobic oxidation to produce nitriles selectively under O2. Aerobic
oxidative conversion of a primary alcohol is also achieved.
J.-H. Noh, J. Kim, J. Org. Chem.,
2015,
80, 11624-11628.

Iron-catalyzed aerobic oxidative reactions of primary amines, secondary amines,
benzylamines with anilines, and alcohols with amines in the presence of air as
the economic and safe oxidant, provide several direct, practical, and greener
approaches for the preparation of useful imines.
E. Zhang, H. Tian, S. Xu, X. Yu, Q. Xu, Org. Lett., 2013,
15, 2704-2707.

The combination of Fe(III), l-valine, and 4-OH-TEMPO catalyzes an oxidation of
alcohols followed by condensation with sulfinamide or sulfonamide in one pot to
provide N-sulfinyl- and N-sulfonyl imines under mild conditions in
very good yields.
G. Zhang, Y. Xing, S. Xu, C. Ring, S. Shan,
Synlett, 2018, 29, 1232-1238.

A Cu/TEMPO catalyst system exhibits both excellent reactivity and
selectivity for the synthesis of alkenylboronates from inexpensive alkenes and
pinacol diboron. This approach enables a direct functionalization of both
aromatic and aliphatic terminal alkenes.
W. Lu, Z. Shen, Org. Lett.,
2019, 21, 142-146.

A wide range of olefins with diverse functionalities has been nitrated in
synthetically useful yields in a single step under metal-free conditions. This
transformation is operationally simple and exhibits excellent E-selectivity.
Furthermore, site selective nitration in a complex setup makes this method
advantageous.
S. Maity, T. Naveen, U. Sharma, D. Maiti, Org. Lett., 2013,
15, 3384-3387.

Ferric nitrate with catalytic TEMPO is a useful combination of reagents for
regio- and stereoselective nitration of various aromatic, aliphatic, and
heteroaromatic olefins. This mild and operationally simple reaction provided
nitroolefins in preparatively useful yields with excellent E-selectivity.
T. Naveen, S. Maity, U. Sharma, D. Maiti, J. Org. Chem., 2013,
78, 5949-5954.

A Cu-catalyzed oxidative amidation-diketonization reaction of terminal alkynes
leads to α-ketoamides. In this copper-catalyzed radical process, O2
not only participates as the ideal oxidant but also undergoes dioxygen
activation under ambient conditions.
C. Zhang, N. Jiao, J. Am. Chem. Soc., 2010,
132, 28-29.

Passerini three-component reaction under catalytic aerobic conditions allows the
conversion of alcohols instead of aldehydes. The reaction of alcohols,
isocyanides, and carboxylic acids in toluene in the presence of a catalytic
amount of cupric chloride, NaNO2, and TEMPO afforded, under an oxygen
atmosphere, the P-3CR adducts in good yields.
J. Brioche, G. Masson, J. Zhu, Org. Lett., 2010,
12, 1432-1435.

α-Oxidation of a variety of carboxylic acids, which preferentially undergo
undesired decarboxylation under radical conditions, proceeded efficiently under
optimized conditions via a chemoselective enolization without stoichiometric
amounts of Brønsted base. The formed redox-active heterobimetallic enediolate
efficiently coupled with free radical TEMPO.
T. Tanaka, R. Yazaki, T. Ohshima, J. Am. Chem. Soc.,
2020, 142, 4517-4524.

Flexible and chemoselective methods for the transition-metal-free oxidation
of amides provide α-keto amides and α-hydroxy amides. These highly valuable
motifs are accessed in good to excellent yields and stereoselectivities with
high functional group tolerance.
A. de la Torre, D. Kaiser, N. Maulide, J. Am. Chem. Soc., 2017,
139, 6578-6581.

A highly convenient organocatalytic method for the mono-oxidation of
unprotected glycosides relies on the chemoselective properties of TEMPO
in combination with trichloroisocyanuric acid under very mild, basic
conditions. The resulting dialdo-glycosides are efficiently purified
with the use of solid-phase imine capture.
M. Angelin, M. Hermansson, H. Dong, O. Ramström, Eur. J. Org. Chem., 2006,
4323-4326.

A mild, aerobic, catalytic synthesis of nitriles directly from alcohols and
aqueous ammonia proceeds via a dehydrogenation cascade mediated by catalytic CuI,
bpy, and TEMPO in the presence of oxygen. The substrate scope includes various
functionalized aromatic and aliphatic alcohols. This protocol also enabled a
one-pot synthesis of various biaryl heterocycles directly from commercially
available alcohols.
W. Yin, C. Wang, H. Huang, Org. Lett., 2013,
15, 1850-1853.

A direct conversion of a wide range of aliphatic, benzylic, heteroaromatic,
allylic, and propargyl alcohols into nitriles with
2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), iodosobenzene diacetate, and
ammonium acetate as a nitrogen source proceeds through an
oxidation-imination-aldimine oxidation sequence in situ. Highly chemoselective
ammoxidation of primary alcohols in the presence of secondary alcohols was also
achieved.
J.-M. Vatèle,
Synlett, 2014, 25, 1275-1278.

An efficient and highly selective method for the oxidative conversion of
primary amines to the corresponding nitriles using trichloroisocyanuric
acid in the presence of catalytic TEMPO provides a new entry to the
synthesis of various aliphatic, aromatic and heterocyclic nitriles in
excellent yield.
F.-E. Chen, Y.-Y. Kuang, H.-F. Dai, L. Lu, M. Huo, Synthesis,
2003, 2629-2631.

Iodine was compared to other positive halogens as terminal oxidant in
chemoselective oxidations of alcohols using catalytic TEMPO and was shown to be
superior in cases of electron-rich and heteroaromatic benzylic alcohols.
R. A. Miller, R. S. Hoerrner, Org. Lett.,
2003, 5, 285-287.

A novel, metal-free oxidation system for the catalytic synthesis of aldehydes
and ketones using TEMPO and a quarternary ammonium salt as catalysts and Oxone
as oxidant proved especially successful for the synthesis of ketones. The mild
conditions tolerate even sensitive silyl protective groups which can otherwise
be cleaved in the presence of Oxone.
C. Bolm, A. S. Magnus, J. P. Hildebrand, Org. Lett., 2000,
2, 1173-1175.

C. Bolm, A. S. Magnus, J. P. Hildebrand, Org. Lett., 2000,
2, 1173-1175.

1 mol-% TEMPO and a catalytic amount of
1,3-dibromo-5,5-dimethylhydantoin and NaNO2 is a highly
efficient catalytic system for the aerobic oxidations of benzylic
alcohols in water.
R. Liu, C. Dong, X. Liang, X. Wang, X. Hu, J. Org. Chem., 2005,
70, 239-244.

Benzylic ethers are oxidatively cleaved by
4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate in wet
MeCN at room temperature to give the corresponding aromatic aldehydes and
alcohols in high yield. Primary and secondary alkyl alcohols are further
oxidized to give carboxylic acids and ketones, respectively.
P. P. Pradhan, J. M. Bobbitt, W. F. Bailey, J. Org. Chem., 2009,
74, 9501-9504.

A smooth, organocatalytic one-pot oxidative cleavage of terminal 1,2-diols to
one-carbon-unit-shorter carboxylic acids is catalyzed by 1-Me-AZADO in the
presence of a catalytica amount of NaOCl and NaClO2 under mild
conditions. A broad range of substrates including carbohydrates and N-protected
amino diols were converted without epimerization.
M. Shibuya, R. Doi, T. Shibuta, S.-i. Uesugi, Y. Iwabuchi, Org. Lett., 2012,
14, 5006-5009.

A copper(II)-catalyzed intermolecular three-component oxyarylation of allenes
using arylboronic acids as a carbon source and TEMPO as an oxygen source
proceeded under mild conditions with high regio- and stereoselectivity and
functional group tolerance.
T. Itoh, Y. Shimizu, M. Kanai, Org. Lett., 2014,
16, 2736-2739.

In situ aerobic dual oxidation with asymmetric organocatalysis enables an
enantioselective synthesis of α-hydrazino aldehydes from alcohols and N-Boc
hydrazine instead of the conventional combination of aldehydes with
azodicarboxylates. This reaction tolerates various substituents on the alcohol
component and features excellent enantiocontrol, cheap starting materials,
operational simplicity, and scalability.
Z. Cui, D.-M. Du, Org. Lett.,
2016, 18, 5616-5619.

Cu/nitroxyl catalysts promote a highly efficient and selective aerobic oxidative
lactonization of diols under mild reaction conditions using ambient air as the
oxidant. A Cu/ABNO catalyst system shows excellent reactivity with symmetrical
diols and hindered unsymmetrical diols, whereas a Cu/TEMPO catalyst system
displays excellent chemo- and regioselectivity for the oxidation of less
hindered unsymmetrical diols.
X. Xie, S. S. Stahl, J. Am. Chem. Soc., 2015,
137, 3767-3770.

Oxoammonium salts enable a practical and highly efficient oxidative
rearrangement of tertiary allylic alcohols to β-substituted α,β-unsaturated
carbonyl compounds. Acyclic substrates as well as medium membered ring
substrates and macrocyclic substrates can be oxidized.
M. Shibuya, M. Tomizawa, Y. Iwabuchi, J. Org. Chem., 2008,
73, 4750-4752.

The use of cheap and innocuous reagents, such as NaClO2, NaOCl,
and catalytic amounts of TEMPO enables an environmentally friendly C(sp3)-H
oxidation of piperazines and morpholines to 2,3-diketopiperazines and
3-morpholinones, respectively. In addition, by using a stoichiometric amount of
TEMPO, 2-alkoxyamino-3-morpholinones can be prepared from morpholine derivatives.
D. Chamorro-Arenas, U. Osorio-Nieto, L. Quintero, L. Hernández-García, F.
Sartillo-Piscil, J. Org. Chem., 2018, 83,
15333-15346.

Microwave-promoted iminyl radical cyclizations can be terminated by trapping
with TEMPO, affording functionalized adducts without using toxic and hazardous
reagents. The use of alkynes as radical acceptors delivers a range of
2-acylpyrroles in good yields.
Y. Cai, A. Jalan, A. R. Kubosumi, S. L. Castle, Org. Lett.,
2015,
17, 488-491.

The use of TEMPO enables a novel, metal-free, and regioselective approach for
the synthesis of isoxazolines or cyclic nitrones substituted with methylene
groups via tandem iminoxyl radical-promoted cyclization or TEMPO-mediated
Cope-like elimination, respectively.
F. Chen, X.-L. Yang, Z.-W. Wu, B. Han, J. Org. Chem.,
2016,
81, 3042-3050.

The use of 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO) as catalyst
enables a Machetti-De Sarlo reaction of nitroalkenes with alkynes/alkenes under
sustainable conditions to afford a library of isoxazole/isoxaline products.
M. Vadivelu, S. Sampath, K. Muthu, K. Karthikeyan, C. Praveen, J. Org. Chem., 2019, 84,
13636-13645.

The combination of gold catalysis and radical chemistry enables the synthesis
of 5-oxazole ketones from internal N-propargylamides in the presence of
4-MeO-TEMPO as an oxidant. The desired 5-oxazole ketones were provided in good
yields with an excellent functional group compatibility under mild conditions.
H. An, S. Mai, Q. Xuan, Y. Zhou, Q. Song, J. Org. Chem., 2019, 84,
401-408.

A simple and efficient cationic Fe(III)/TEMPO-catalyzed oxidative cyclization
of aroyl hydrazones in the presence of oxygen enables the synthesis of 2,5-disubstituted
1,3,4-oxadiazole derivatives in high yields. The reaction offers a broad scope
and good functional-group tolerance.
G. Zhang, Y. Yu, Y. Zhao, X. Xie, C. Ding,
Synlett, 2017, 28, 1373-1377.

An operationally simple, regioselective reaction of ketones, aldehydes, or
esters with amidines in the presence of TEMPO and an in situ prepared recyclable
iron(II)-complex provides various pyrimidine derivatives with broad functional
group tolerance. The reactions are likely to proceed through a TEMPO
complexation/enamine addition/transient α-occupation/β-TEMPO
elimination/cyclization sequence.
X.-Q. Chu, W.-B. Cao, X.-P. Xu, S.-J. Ji, J. Org. Chem.,
2017, 82, 1145-1154.

A Cu-catalyzed and 4-HO-TEMPO-mediated [3 + 3] annulation of commercially
available amidines with saturated ketones enables an efficient and facile
synthesis of structurally important pyrimidines via a cascade reaction of
oxidative dehydrogenation/annulation/oxidative aromatization.
J.-L. Zhang, M.-W. Wu, F. Chen, B. Han, J. Org. Chem.,
2016, 81, 11994-12000.

Oxidative one-pot sequential reactions of inactivated saturated ketones with
electron-deficient enamines enable an efficient synthesis of 3-acylpyridines and
pyridine-3-carboxylates. The reaction involve oxidative dehydrogenation of the
saturated ketone substrate, followed by [3+3] annulation with β-enaminone or
β-enaminoester via a cascade process, including Michael addition, aldol type
condensation, and oxidative aromatization.
G. Chen, Z. Wang, X. Zhang, X. Fan, J. Org. Chem.,
2017, 82, 11230-11237.

An efficient oxidative protocol enables the synthesis of multisubstituted or
fused tetracyclic benzimidazoles via a metal-free oxidative C-N coupling between
the sp3 C-H and free N-H of readily available N1-benzyl/alkyl-1,2-phenylenediamines
in the presence of oxygen and TEMPO.
D. Xue, Y.-Q. Long, J. Org. Chem., 2014,
79, 4727-4734.

A visible-light-driven, intramolecular C(sp2)-H thiolation
without addition of a photosensitizer, metal catalyst, or base
induces the cyclization of thiobenzanilides to benzothiazoles. The substrate undergoes a reverse hydrogen-atom
transfer (RHAT) in its excited state with TEMPO to form a sulfur
radical, which adds to the benzene ring followed by a rearomatization via RHAT.
Z.-M. Xu, H.-X. Li, D. J. Young, D.-L. Zhu, H.-Y. Li, J.-P. Lang, Org. Lett.,
2019, 21, 237-241.

A one-pot method enables the synthesis of multisubstituted indolizines from
α-halo carbonyl compounds, pyridines, and electron-deficient alkenes via
oxidative dehydrogenation under transition-metal-free conditions using TEMPO as
an oxidant. This protocol uses readily available starting materials in a
convenient procedure under mild reaction conditions.
F. Shi, Y. Zhang, Z. Lu, X. Zhu, W. Kan, X. Wang, H. Hu,
Synthesis, 2016, 48, 413-420.

A TEMPO oxoammonium salt mediates a metal-free oxidative dearomatization of
indoles with aromatic ketones in the presence of H2SO4 to
provide 2,2-disubstituted indolin-3-ones in good yields. The dearomatization
proceeds smoothly and displays a broad substrate scope with respect to both
indoles and aromatic ketones.
J. Liu, J. Huang, K. Jia, T. Du, C. Zhao, R. Zhu, X. Liu, Synthesis, 2020, 52,
763-768.

CuCl/DABCO/4-HO-TEMPO as the catalysts and oxygen as the terminal oxidant
enabled an efficient aerobic oxidative synthesis of 2-substituted quinazolines
and 4H-3,1-benzoxazines from the one-pot reaction of aldehydes with
2-aminobenzylamines and 2-aminobenzyl alcohols, respectively.
B. Han, X.-L. Yang, C. Wang, Y.-W. Bai, T.-C. Pan, X. Chen, W. Yu, J. Org. Chem., 2012,
77, 1136-1142.

An efficient copper-catalyzed cascade reaction of (2-aminophenyl)methanols with
aldehydes using the combination of cerium nitrate hexahydrate and ammonium
chloride leads to a wide range of 2-substituted quinazolines in good yields. The
method tolerates a various functional groups and represents a convenient and
practical strategy for synthesis of 2-substituted quinazoline derivatives.
Z. Chen, J. Chen, M. Liu, J. Ding, W. Gao, X. Huang, H. Wu, J. Org. Chem., 2013,
78, 11342-11348.

A fast and simple reaction of amidines gave benzimidazoles via
iodine(III)-promoted oxidative C(sp3)-C(sp2) bond
formation in nonpolar solvents, whereas the use of polar solvents favoured a
C(sp2)-N bond formation to yield quinazolines. Further selective
synthesis of quinazolines in polar solvent was realized using TEMPO as catalyst
and K2S2O8 as the oxidant. No metal, base, or
other additives were needed.
J.-P. Lin, F.-H. Zhang, Y.-Q. Long, Org. Lett., 2014,
16, 2822-2825.

A one-pot dehydrogenative Povarov/oxidation tandem reaction of N-alkyl anilines
with mono- and 1,2-disubstituted aryl and alkyl olefins enables the synthesis of
a various substituted quinolines. The simple protocol uses cheap and benign
iron(III)chloride as the Lewis acid catalyst and a TEMPO oxoammonium salt as a
nontoxic, mild, efficient oxidant.
H. Richter, O. G. Mancheño, Org. Lett., 2011,
13, 6066-6069.

Using TEMPO as the oxidant and KOtBu as the base enables a metal-free, a
simple and direct access to a broad range of 2-arylquinolin-4(1H)-ones
from readily available N-arylmethyl-2-aminophenylketones via oxidative
intramolecular Mannich reaction.
W. Hu, J.-P. Lin, L.-R. Song, Y.-Q. Long, Org. Lett.,
2015,
17, 1268-1271.

Cross coupling of ortho-substituted aryl Grignard reagents with
alkynyl Grignard reagents can be performed without adding any transition metal
in the presence of 2,2,6,6-tetramethylpiperidine-N-oxyl radical (TEMPO)
as an environmentally benign organic oxidant. Importantly, functional groups
such as esters, amides, and cyanides are tolerated.
M. S. Maji, S. Murarka, A. Studer, Org. Lett., 2010,
12, 3878-3881.

A method for aliphatic C-H bond oxidation of oximes and hydrazones mediated by
2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO) enables the concise assembly of
substituted isoxazole and pyrazole skeletons.
X. Zu, Y.-F. Wang, W. Ren, F.-L. Zhang, S. Chiba, Org. Lett., 2013,
15, 3214-3214.

An easily prepared recyclable TEMPO derived sulfonic salt catalyst, and
mineral acids (NaNO2 and HCl) enable a selective aerobic oxidation of
structurally diverse benzylic sp3 C-H bonds of ethers and alkylarenes
to provide synthetically and biologically valued isochromanones and xanthones in
good yields.
Z. Zhang, Y. Gao, Y. Liu, J. Li, H. Xie, H. Li, W. Wang, Org. Lett.,
2015,
17, 5492-5495.
