tert-Butyl hydroperoxide, TBHP
Name Reactions
Recent Literature

Various aromatic, aliphatic and conjugated alcohols were transformed into the
corresponding carboxylic acids and ketones in good yields with aq 70% t-BuOOH
in the presence of catalytic amounts of bismuth(III) oxide. This method
possesses does not involve cumbersome work-up, exhibits chemoselectivity and
proceeds under ambient conditions. The overall method is green.
P. Malik, D. Chakraborty, Synthesis, 2010, 3736-3740.

The use of tert-butyl hydroperoxide (TBHP) as a terminal oxidant in the
presence of catalytic amount of tetrabutylammonium iodide and imidazole enables
a transition-metal-free synthesis of aryl esters in high yield starting from
benzylic primary alcohols and aliphatic alcohols. These reactions are highly
chemoselective and tolerate a wide range of substituents.
S. Nandy, A. Ghatak, A. K. Das, S. Bhar, Synlett, 2018, 29,
2208-2212.

A highly effective synthesis of methyl esters from benzylic alcohols, aldehydes,
or acids via copper-catalyzed C-C cleavage from tert-butyl hydroperoxide
is easily accessible and practical and offers an alternative to the traditional
way.
Y. Zhu, H. Yan, L. Lu, D. Liu, G. Rong, J. Mao, J. Org. Chem., 2013,
78, 9898-9905.

A highly effective synthesis of methyl esters from benzylic alcohols, aldehydes,
or acids via copper-catalyzed C-C cleavage from tert-butyl hydroperoxide
is easily accessible and practical and offers an alternative to the traditional
way.
Y. Zhu, H. Yan, L. Lu, D. Liu, G. Rong, J. Mao, J. Org. Chem., 2013,
78, 9898-9905.

A copper-catalyzed oxidative cleavage reaction of terminal and internal
alkynes using NFSI and TBHP provides aryl ketone products in good yields. NFSI
not only functioned as N-centered radical precursor but also engaged in
the aryl group migration. Mechanistic studies also suggested the important role
of water.
L. Tang, F. Yang, H. Cheng, C. Tan, C. Jin, H. Chen, Y. Huang, S. Zhang, S.
Zhang, W. Song, J. Tan,
Org. Lett., 2020, 22, 8618-8623.

The combination of copper(I) iodide and an N-heterocyclic carbene catalyzes an
oxidative amidation of aldehydes with amines in the presence of tert-butyl
hydroperoxide. The method is simple and practicable, has a broad substrate
scope, and uses economical, feasible, and abundant reagents.
A. Singh, A. K. Narula, Synlett, 2021, 32,
718-722.
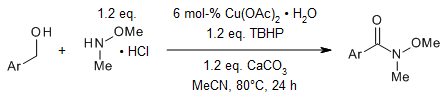
The use of tert-butyl hydroperoxide as an oxidant and an inexpensive and
air stable copper catalyst enables a simple and efficient protocol for the
oxidative amidation of commercially affordable alcohols to Weinreb amides in
very good yields. The reaction tolerates various functional groups.
S. L. Yedage, B. M. Bhanage, Synthesis, 2015, 47,
526-532.

Using an efficient visible-light photocatalysis-based method, a mixture of an
aldehyde, tert-butyl hydrogen peroxide, and N-chlorosuccinimide
afforded an acid chloride in the presence of Ru(bpy)3Cl2
as photocatalyst. A subsequent reaction with an amine provided the corresponding
amide.
N. Iqbal, E. J. Cho, J. Org. Chem.,
2016,
81, 1905-1911.

Ru complexes with tridentate hybrid ligands showed a very strong catalytic
activity in water at room temperature for benzylic C-H oxidation. Whereas NHCs
with a stronger donor ability stabilize the Ru center; nitrogen ligands with a
relatively weaker donor ability release from the Ru center, so that they induce
a reaction.
C.-B. Bo, Q. Bu, X. Li, G. Ma, D. Wei, C. Guo, B. Dai, N. Liu, J. Org. Chem., 2020, 85,
4324-4334.

The cationic complex [(pymox-Me2)RuCl2]+BF4-
is a highly effective catalyst for the C-H bond oxidation of aryl alkanes in
water using tert-butyl
hydroperoxide as oxidant to yield various aryl ketones at room temperature in
water as solvent. A solvent-caged oxygen rebounding mechanism via a Ru(IV)-oxo
intermediate species is suggested.
C. S. Yi, K.-H. Kwon, D. W. Lee, Org. Lett., 2009,
11, 1567-1569.

The bismuth and picolinic acid-catalyzed oxidation of alkyl arenes with
tert-butyl hydroperoxide in pyridine and acetic acid gave benzylic
ketones in good yields. Alternatively, oxidation of methyl arenes gave the
corresponding substituted benzoic acids. A radical mechanism is discussed.
Y. Bonvin, E. Callens, I. Larrosa, D. A. Henderson, J. Oldham, A. J. Burton,
A. G. M. Barrett, Org. Lett.,
2005, 7, 4549-4552.

Copper(II) catalyzes a cross dehydrogenative coupling (CDC) reaction of
aldehydes with alkylbenzenes in the presence of TBHP to yield benzylic esters.
S. K. Rout, S. Guin, K. K. Ghara, A. Banerjee, B. K. Patel, Org. Lett., 2012,
14, 3982-3985.

In an unusual oxidative coupling reaction of isocyanide and toluene derivatives
using tetrabutylammonium iodide (TBAI) as a catalyst, the isocyano group acts
formally as an N1 synthon, thus expanding the reactivity profile of isocyanides.
Z. Liu, X. Zhang, J. Li, F. Li, C. Li, X. Jia, J. Li, Org. Lett.,
2016, 18, 4032-4035.

A metal-free and one-pot two-step synthesis of aryl carboxylic acids from aryl
alkyl ketones has been performed with iodine as the catalyst, DMSO and TBHP as
the oxidants. Various aryl alkyl ketones could be converted into their
corresponding aryl carboxylic acids in very good yields.
L. Xu, S. Wang, B. Chen, M. Li, X. Hu, B. Hu, L. Jin, N. Sun, Z. Shen,
Synlett, 2018, 29, 1505-1509.

An efficient, metal-free domino protocol for the synthesis of benzamides from
ethylarenes proceeds through the formation of triiodomethyl ketone intermediate
in the presence of iodine as the promoter and TBHP as an oxidant followed by
nucleophilic substitution with aqueous ammonia. This operationally simple,
functional-group-tolerant tandem approach provides an easy access to the broad
range of biologically important benzamides.
K. S. Vadagaonkar, H. P. Kalmode, S. Prakash, A. C. Chaskar,
Synlett, 2015, 26, 1677-1682.

A copper-catalyzed cross-coupling reaction of amines with alkoxycarbonyl
radicals generated from carbazates provides carbamates under mild conditions.
This environmentally friendly approach is compatible with a wide range of
amines, including aromatic/aliphatic and primary/secondary substrates.
S.-N. Wang, G.-Y. Zhang, A. Shoberu, J.-P. Zou, J. Org. Chem., 2021, 86,
9067-9075.

A highly efficient oxidation of propargylic alcohols to ynones is catalyzed by
copper nanoparticles (Cu Nps) with TBHP or air as oxidants. With bipyridine as
the ligand, the reaction was accelerated significantly and led in good to
excellent yields to a variety of propargylic alcohols.
C. Han, M. Yu, W. Sun, Y. Yao, Synlett, 2011,
2363-2368.

Pd-Catalyzed TBHP-Mediated Selective Wacker-Type Oxidation and
Oxo-acyloxylation of Olefins Using a 2-(1H-Indazol-1-yl)quinoline Ligand
S. Zhang, J. Zhang, H. Zou, Org. Lett., 2023, 25,
1850-1855.

A practical and environmentally friendly oxidation of aryl olefins to
arylethanone derivatives by using a Cu(I) catalyst and tert-butyl
hydroperoxide (TBHP) provides 2-tert-butoxy-1-arylethanones in good
yields under mild conditions with high selectivity. In this method, TBHP acts
not only as an oxidant but also as the tert-butoxy and carbonyl oxygen
sources.
J. Zhang, D. Xiao, H. Tan, W. Liu, J. Org. Chem., 2020, 85,
3929-3935.

Iodine catalyzes an environment-friendly and efficient dioxygenation of aryl
alkenes for the construction of vicinal diols in water as solvent with tert-butylhydroperoxide
(TBHP) as the oxidant. The protocol is efficient, sustainable, and operationally
simple. In addition, bisperoxides could be obtained in good yields with Na2CO3
as additive and propylene carbonate as solvent.
X. Gao, J. Lin, L. Zhang, X. Luo, G. Guo, N. Peng, H. Xu, Y. Liu, J. Org. Chem., 2021, 86,
15469-15480.

Iodine catalyzes an environment-friendly and efficient dioxygenation of aryl
alkenes for the construction of vicinal diols in water as solvent with tert-butylhydroperoxide
(TBHP) as the oxidant. The protocol is efficient, sustainable, and operationally
simple. In addition, bisperoxides could be obtained in good yields with Na2CO3
as additive and propylene carbonate as solvent.
X. Gao, J. Lin, L. Zhang, X. Luo, G. Guo, N. Peng, H. Xu, Y. Liu, J. Org. Chem., 2021, 86,
15469-15480.

TBAI catalyzes an efficient, mild, and regioselective synthesis of
4-aryl/alkyl-1-peroxy-but-3-en-2-ols in good yields from
1-substituted-1,3-butadienes in the presence of hydroperoxides and water. This
regioselective orthogonal dioxygenation of a diene can be executed in a simple
operation and tolerates a wide range of substrates.
A. Kumar, G. N. Khatun, R. A. Fernandes, Org. Lett., 2023, 25,
4313-4317.

Chiral amino acid-based hydroxamic acids can be effective asymmetric catalysts
for the epoxidation of allylic alcohols, especially disubstituted allylic
alcohols. The mild reaction conditions, e.g., reasonable temperature, low degree
of catalyst loading, and halogen-free solvent, extend the scope of this process.
Y. Hoshino, H. Yamamoto, J. Am. Chem. Soc., 2000,
122, 10452-10453.

A simple and efficient enantioselective epoxidation of α,β-unsaturated ketones
is catalyzed by rare-earth metal amides in the presence of
phenoxy-functionalized chiral prolinols at room temperature using tert-butylhydroperoxide
(TBHP) as the oxidant. The combination of an Yb-based amide and a chiral
proligand provided chiral epoxides in excellent yields and enantiomeric excess
of up to 99%.
C. Zeng, D. Yuan, B. Zhao, Y. Yao, Org. Lett.,
2015,
17, 2242-2245.

Heterobimetallic complexes stabilized by chiral phenoxy-functionalized
prolinolate are highly active in catalyzing the epoxidation of α,β-unsaturated
ketones, while the enantioselectivity varies according to the ionic radii of the
rare earth center. A series of chalcone derivatives were converted to chiral
epoxides in good ee at 0°C using TBHP as the oxidant.
Q. Qian, Y. Tan, B. Zhao, T. Feng, Q. Shen, Y. Yao, Org. Lett.,
2014,
16, 4516-4519.

Under the synergistic actions of photocatalyst Ru(bpy)3Cl2,
tert-butyl hydroperoxide, cesium carbonate, and visible light irradiation,
a range of styrenes and benzaldehydes smoothly form α,β-epoxy ketones via
visible-light-enabled photocatalytic generation of acyl radicals as key
intermediates.
J. Li, D. Z. Wang, Org. Lett.,
2015,
17, 5260-5263.

Utilizing the rapidly synthesized Quinox ligand and commercially available
aqueous TBHP, a Wacker-type oxidation efficiently converts even traditionally
challenging substrates such as protected allylic alcohols to the corresponding
oxidized products. Enantioenriched substrates undergo oxidation with complete
retention of enantiomeric excess.
B. W. Michel, A. M. Camelio, C. N. Cornell, M. S. Sigman, J. Am. Chem. Soc., 2009,
131, 6076-6077.

Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP
R. J. DeLuca, J. L. Edwards, L. D. Steffens, B. W. Michel, X. Qiao, C. Zhu, S.
P. Cook, M. S. Sigman, J. Org. Chem., 2013,
78, 1682-1683.

I2-catalyzed oxo-acyloxylation of alkenes and enol ethers with
carboxylic acids provides α-acyloxyketones and esters in high yields. This
unprecedented regioselective oxidative process employs TBHP and Et3N
in stoichiometric amounts under metal-free conditions in DMSO as solvent.
α-Acyloxyketones can be converted in situ to monoprotected diol derivatives in
excellent yields upon treatment with BH3ˇSMe2.
R. N. Reddi, P. K. Prasad, A. Sudalai, Org. Lett.,
2014,
16, 5674-5677.

A cobalt-catalyzed
α-methoxymethylation of ketones with methanol as a sustainable C1 source,
cheap CoCl2ˇ6H2O as catalyst and TBHP as oxidant provides the methoxymethylated products
within a short reaction time in very good yield. α-Aminomethylated ketones can
be produced by a one-pot methylenation/aza-Michael addition sequence or by a
base-mediated conversion of the α-methoxymethyl ketones.
J. Yang, S. Chen, H. Zhou, C. Wu, B. Ma, J. Xiao, Org. Lett.,
2018, 20, 6774-6779.

An sp3 C-H bond-transformation reaction of methylarenes provides the
corresponding allylbenzene derivatives in good yields in the presence of
tetrabutylammonium iodide and tert-butyl hydroperoxide at 80 °C.
F. Shahsavari, A. Abbasi, M. Ghaznafarpour-Darjjani, S. M. Ghafelebashi, M.
Daftari-Besheli,
Synlett, 2017, 28, 1646-1648.

A ruthenium-catalyzed oxidation of alkenes allows an efficient route to
α-diketones using TBHP as an oxidant, is highly functional group tolerant and
practically convenient, requires no additional ligand, and operates under mild
conditions with short reaction times. Based upon experimental observations, a
plausible mechanism is proposed.
S. Chen, Z. Liu, E. Shi, L. Chen, W. Wei, H. Li, Y. Cheng, X. Wan, Org. Lett., 2011,
13, 2274-2277.

A concerted metallophotoredox catalysis enables an efficient decarboxylative
functionalization of α,β-unsaturated carboxylic acids with aryl iodides in the
presence of perylene bisimide dye to afford 1,2-diketones.
S. Chand, A. K. Pandey, R. Singh, K. N. Singh, J. Org. Chem., 2021, 86,
6343-6350.

A recyclable, bifunctional iron nanocomposite catalyzes an efficient synthesis
of α-keto acids via oxidation of alkenes using TBHP as oxidant. A variety of
alkenes with different functional groups were smoothly oxidized into their
corresponding α-keto acids in good yields.
T. Song, Z. Ma, X. Wang, Y. Yang, Org. Lett., 2021, 23,
5917-5921.

A copper-catalyzed one-pot strategy for the synthesis of α-ketoamides from
1-arylethanols is highly efficient and delivers product in very good yields via
alcohol oxidation, sp3 C-H oxidation, and oxidative amidation.
N. Sharma, S. S. Kotha, N. Lahiri, G. Sekar, Synthesis, 2015, 47,
726-736.

A simple and efficient oxidative coupling of diazoesters and α-diazoketones
with NH4I provides primary oxamates and α-ketoamides in good yields. This metal-free protocol is performed
under mild conditions and has a wide substrate scope.
H. Wang, Y. Zhao, Y. Zheng, S. Fang, J. Li, X. Wan, J. Org. Chem., 2020, 85,
3050-3058.

The reaction of sulfoxonium ylides with primary or secondary amines afforded
α-ketothioamides in the presence of elemental sulfur, whereas α-ketoamides were
produced when I2 and TBHP were present. This simple, scalable
reaction proceeded well at room temperature, tolerated a range of functional
group, and generated the corresponding products in very good yields.
T. N. Chaubey, P. J. Borpatra, A. Sharma, S. K. Pandey, Org. Lett., 2022, 24,
8062-8066.

In a Co-catalyzed reaction for the construction of 1,4-dicarbonyls, a cascade
organocobalt addition/trapping/Kornblum-DeLaMare rearrangement were involved.
The reaction offers easy availability of starting materials, wide substrate
scope, high functionality tolerance, and operational simplicity.
F. Zhang, P. Du, J. Chen, H. Wang, Q. Luo, X. Wan, Org. Lett., 2014,
16, 1932-1935.

In the presence of TBAI/TBHP, treatment of esters possessing a methylene carbon
α-to oxygen with benzylamines provides bis-esters rather than the expected
amides. Under these oxidative conditions, benzylamines generate less
nucleophilic carboxylates, which couple at sp3 C-H bonds of esters
and cyclic ethers to yield bis-acyl ketals and α-acyloxy ethers, respectively.
G. Majji, S. Rajamanickam, N. Khatun, S. K. Santra, B. K. Patel, J. Org. Chem.,
2015,
80, 3440-3446.

An efficient and modular strategy based on a four-component sequential
reaction provides enaminones under mild conditions without a catalyst
in one pot. Furthermore, the products could be transformed into thiadiazoles.
J. Zhang, P. Zhou, A. Yin, S. Zhang, W. Liu, J. Org. Chem., 2021, 86,
8980-8986.

A copper-based system enables methylene insertion between an amine and an
alkyne counterpart, via C-N bond cleavage of N,N-dimethylacetamide. The
method gives an expedient access to a broad range of propargylic amines in good
yields.
A. Rahaman, R. D. Shinde, S. Bhadra, J. Org. Chem., 2023, 88,
1884-1889.

Regiospecific radical reactions of β-alkyl nitroalkenes with sulfonyl hydrazides
provide allyl sulfones with high regioselectivity in the presence of
dimethylformamide (DMF), whereas reactions in acetonitrile provide vinyl
sulfones.
Y. Wang, G. Xiong, C. Zhang, Y. Chen, J. Org. Chem., 2021, 86,
4018-4026.

A regio- and stereoselective copper-catalyzed sulfonylation of alkynyl imines
with sulfonyl hydrazides provides a series of (E)-β-sulfonyl enones in
good yields. Mechanistic studies suggest a
radical process.
R. Chen, S. Li, J. Zhang, J. Cao, K.-K. Wang, T. Meng, L. Liu, J. Org. Chem., 2022, 87,
13322-13330.

A facile photocatalyzed strategy for difunctionalization of styrenes in the
presence of CS2 and amines provides β-keto dithiocarbamates. However,
4-nitrostyrene and 2-vinylpyridine can only be converted to
2-arylethylthiocarbamates.
R. K. Vishwakarma, S. Kumar, K. N. Singh, Org. Lett., 2021, 23,
4147-4151.

Olefin substrates can be converted to the corresponding enones or 1,4-enediones
in very good yields in short reaction times using a Cu(II) 2-quinoxalinol salen
complex as the catalyst and tert-butyl hydroperoxide (TBHP) as the
oxidant via allylic activation. The reaction tolerates many additional
functional groups.
Y. Li, T. B. Lee, T. Tang, A. V. Gamble, A. E. V. Gorden, J. Org. Chem., 2012,
77, 4628-4633.

Dirhodium(II) caprolactamate effectively catalyzes the allylic oxidation of
a variety of olefins and enones with tert-butyl hydroperoxide as
terminal oxidant. The reaction is completely selective, tolerant of air and
moisture, and can be performed with as little as 0.1 mol % catalyst in
minutes.
A. E. Lurain, A. Maestri, A. R. Kelli, P. J. Carroll, P. J. Walsh, J. Am. Chem. Soc.,
2004,
126, 13622-13623.

The allylic oxidation of cyclic alkenes with a copper-aluminum mixed oxide as
catalyst in the presence of a carboxylic acid and tert-butyl
hydroperoxide as the oxidant gives the corresponding allylic esters. When
l-proline is employed, the allylic alcohol or ketone is obtained.
A. L. García-Cabeza, R. Marín-Barrios, F. J. Moreno-Dorado,
M. J. Ortega, G. M. Massanet, F. M. Guerra, Org. Lett., 2014,
16, 1598-1601

A new and simple method is described for the one-step oxidation of α,β-enones
to 1,4-enediones in good yields using t-butylhydroperoxide as
stoichiometric oxidant and 20% Pd(OH)2 on carbon as catalyst. The same reagents have been used to convert ethylene ketals
of α,β-enones
to the corresponding monoethylene ketals of 1,4-enediones. The mechanism is
discussed.
J.-Q. Yu, E. J. Corey, J. Am. Chem. Soc., 2003,
125, 3232-3233.

A new catalytic system for the asymmetric epoxidation of allylic alcohols
has been developed featuring high enantioselectivity for Z olefins,
catalyst loading of less than 1 mol%, reaction temperatures of 0°C to room
temperature over a shorter time, use of aqueous tert-butyl
hydroperoxide (TBHP) instead of anhydrous TBHP as an achiral oxidant, and
simple workup procedures for small expoxy alcohols.
W. Zhang, A. Basak, Y. Kosugi, Y. Hoshino, H. Yamamoto, Angew. Chem. Int. Ed.,
2005,
44, 4389-4391.

An operationally straightforward method for the amidation of aldehydes with
economic ammonium chloride or amine hydrochloride salts enables the synthesis of
various amides in good yield by using inexpensive copper sulfate or copper(I)
oxide as a catalyst and aqueous tert-butyl hydroperoxide as an oxidant.
Chiral amines can be used without detectable racemization.
S. C. Ghosh, J. S. Y. Ngiam, A. M. Seayad, D. T. Tuan, C. L. L. Chai, A. Chen, J. Org. Chem., 2012,
77, 8007-8015.

A mild and efficient oxidative amidation of aldehydes uses amine HCl salts
and tert-butyl hydroperoxide as an oxidant in the presence of a
copper catalyst.
W.-J. Yoo, C.-J. Li, J. Am. Chem. Soc., 2006,
128, 13064-13065.

A general and efficient method enables the synthesis of tertiary amides from
readily available tertiary amines and anhydrides in the presence of FeCl2
as catalyst and tert-butyl hydroperoxide in water (T-Hydro) as oxidant.
Mechanistic studies indicated that the in situ-generated α-amino peroxide of
tertiary amine and iminium ion act as key intermediates.
Y. Li, L. Ma, F. Jia, Z. Li, J. Org. Chem., 2013,
78, 5638-5646.

A direct coupling of NH-amides with methylarenes under iodine/aqueous TBHP
conditions enables a metal-free synthesis of imides. The optimized conditions
worked also very well with benzaldehydes and benzyl alcohol and furnished the
corresponding imides in good to excellent yields.
H. Aruri, U. Singh, S. Kumar, M. Kushwaha, A. P. Gupta, R. A. Vishwakarma, P. P.
Singh, Org. Lett.,
2016, 18, 3638-3641.

A new α-amino acid esters formation via decarboxylation is distinguished by
readily accessible starting materials, environmentally benign reaction
conditions and waste streams, and wide substrate scope.
J. Zhang, J. Jiang, Y. Li, Y. Zhao, X. Wang, Org. Lett., 2013,
15, 3222-3225.

S-oxidation of potassium thiocyanate releases cyanide units that can be
trapped in the presence of co-oxidized tertiary amines to form α-amino nitriles.
These cyanations work in aqueous solutions without catalyst and without toxic
byproducts.
A. Wagner, A. R. Ofial, J. Org. Chem.,
2015,
80, 2848-2854.

An efficient, safe, and environmentally friendly TBHP-mediated rearrangement
of aryl/alkylidene malononitrile with anilines produces in situ HCN as the
cyanide source for the synthesis of substituted α-aminonitriles and
α-aminoamides in very good yields. This method features good functional group
tolerance, and the in situ-generated HCN bypasses the use of an external cyanide
source.
S. P. Bhoite, A. H. Bansode, G. Suryavanshi, J. Org. Chem., 2020, 85, 14858-14865.

TBAI as catalyst and TBHP as oxidant enable a metal-free cross-coupling of
enamines and electron-deficient amines through oxidative C(sp2)-N
bond formation. This efficient organocatalytic synthesis of synthetically useful
diaminoalkene derivatives features readily available starting materials and wide
substrate scope.
Y. Yuan, W. Hou, D. Zhang-Negrerie, K. Zhaou, Y. Du, Org. Lett.,
2014,
16, 5410-5113.

An iron-catalyzed acyl-azidation of alkenes provides unsymmetrical β-azido
ketones under mild reaction conditions from aromatic aldehydes or aliphatic aldehydes as
the acyl radical precursors, TMSN3 as the azido source, and TBHP as
the initiator. The synthesized unsymmetrical β-azido ketones can be easily
transformed into valuable functionalized compounds.
L. Ge, Y. Li, H. Bao, Org. Lett.,
2019, 21, 256-260.

An oxidative copper-catalyzed arylation of various ring-size lactams with
arylboronic acids gives N-arylated products in good yield without any additional
bases, ligands, or additives.
T. Bathini, V. S. Rawat, B. Sreedhar,
Synlett, 2015, 26, 1348-1351.

The catalytic asymmetric addition of alkyl groups to ketones under highly
concentrated and solvent-free conditions permits reduction in catalyst loading
by a factor of 2- to 40-fold compared with standard reaction conditions
employing toluene and hexanes. Using cyclic conjugated enones, solvent-free
asymmetric addition followed by a diastereoselective epoxidation using 5.5 M
decane solution of tert-butyl hydroperoxide generated epoxy alcohols.
S.-J. Jeon, H. Li, P. J. Walsh, J. Am. Chem. Soc.,
2005,
127, 16416-16425.

A catalytic asymmetric epoxidation reaction of various α,β-unsaturated
esters via a conjugate addition of an oxidant using an yttirium-chiral
biphenyldiol catalyst yielded the corresponding α,β-epoxy esters in up to
97% yield and 99% ee.
H. Kakei, R. Tsuji, T. Ohshima, M. Shibasaki, J. Am. Chem. Soc.,
2005,
127, 8962-8963.

A gold(I)-catalyzed oxidative cleavage of alkenes with tert-butyl
hydrogenperoxide (TBHP) as the oxidant in the presence of neocuproine
afforded ketones or aldehydes as products.
D. Xing, B. Guan, G. Cai, Z. Fang, L. Yang, Z. Shi, Org. Lett.,
2006,
8, 693-696.

With an easily accessible cinchona alkaloid catalyst, efficient enantioselective
peroxidation and epoxidation have been successfully developed. Employing readily
available α,β-unsaturated ketones and hydroperoxides, this novel reaction will
open new possibilities in the asymmetric synthesis of chiral peroxides and
epoxides.
X. Lu, Y. Liu, B. Sun, B. Cindric, L. Deng, J. Am. Chem. Soc., 2008,
130, 8134-8135.

Stereodefined enol derivatives of aldehydes are prepared from terminal alkynes
through Cp2ZrCl2-catalyzed methylalumination and
subsequent oxygenation with peroxyzinc species and electrophilic trapping with
carboxylic anydrides. The tandem carbometalation/oxygenation tolerates free and
protected alcohols, heterocycles, olefins, and nitriles.
J. R. DeBergh, K. M. Spivey, J. M. Ready, J. Am. Chem. Soc., 2008,
130, 7828-7829.

A mild and efficient protocol for the synthesis of phenols from arylboronic
acids in the presence of tert-butyl hydroperoxide is promoted by KOH.
Products were obtained in good to excellent yields within several minutes.
S. Guo, L. Lu, H. Cai, Synlett, 2013, 24,
1712-1714.

A copper iodide mediated cyanation of arylboronic acids and aryl iodides with
ethyl (ethoxymethylene)cyanoacetate as cyanating agent involves a C(sp2)-CN
bond cleavage and tolerates a wide range of functional groups to provide the
corresponding aryl nitriles in good yields.
C. Qi, X. Hu, H. He,
Synlett, 2016, 27, 1979-1982.

Nitroarenes react with anions of tert-butyl and cumyl
hydroperoxides in the presence of strong bases to form substituted o- and
p-nitrophenols. The reaction usually proceeds in high yields and is of
practical value as a method of synthesis and manufacturing of nitrophenols.
M. Makosza, K. Sienkiewicz, J. Org. Chem., 1998,
63, 4199-4208.

In a mild, iodide-catalyzed process to synthesize N-nitrosamines from
amines and nitromethane using TBHP as the oxidant, the catalytic system
succeeded in cleaving the carbon-nitrogen bond in nitromethane. This methodology
uses commercially available, inexpensive catalysts and oxidants and has a wide
substrate scope and operational simplicity.
J. Zhang, J. Jiang, Y. Li, X. Wan, J. Org. Chem., 2013,
78, 11366-11372.

A simple and effective copper-catalyzed oxidative cross-coupling of
dimethylanilines with alkynes in the presence of tert-BuOOH allows
the construction of propargylamines via a combination of sp3 C-H
bond and sp C-H bond activations followed by C-C bond formation.
Z. Li, C.-J. Li, J. Am. Chem. Soc.,
2004,
126, 11810-11811.

A mild, scalable iodine-mediated oxidative cross-coupling reaction of
arylhydrazines and thiols enables the construction of thioethers (sulfides) in
the absence of any transition metals or photocatalysts. A variety of
unsymmetrical diaryl sulfides with broad substrate scope both on thiols and
hydrazines were synthesized in high yields in water at room temperature.
F. Jafarpour, M. Asadpour, M. Azizzade, M. Ghasemi, S. Rajai-Daryasarei, Synthesis, 2020, 52,
727-734.

The dirhodium(II) carboxylate complex Rh2(esp)2 catalyzes
the sulfoxidation of organic sulfides in the presence of tert-butyl
hydroperoxide as the oxidant. As the rhodium catalyst is able to precipitate as
a Rh2(esp)2-sulfoxide complex following the reaction, its
separation and reuse is very convenient without considerable loss of activity.
L. Zhao, H. Zhang, Y. Wang, J. Org. Chem.,
2016,
81, 129-134.

An oxidative variant of the thiol-ene reaction enables the direct addition of
thiols to olefins to form sulfoxides in the presence of tert-butyl
hydroperoxide as oxidant and methanesulfonic acid as catalyst. The latter is
believed to catalyze the oxidation of the intermediate sulfide to the sulfoxide.
Styrenes, acrylic acid derivatives, alkynes, and thiophenols gave the highest
yields, while aliphatic olefins and thiols were less effective.
H.-L. Yue, M. Klussmann,
Synlett, 2016, 27, 2505-2509.

A metal-free room temperature decarboxylative cross-coupling between cinnamic
acids and arylsulfonyl hydrazides provides (E)-vinyl sulfones. A regio-
and stereoselective synthesis of 22 derivatives with diverse structural features
has been achieved.
R. Singh, B. K. Allam, N. Singh, K. Kumari, S. K. Singh, K. N. Singh, Org. Lett.,
2015,
17, 2656-2659.

A highly efficient, metal-free, and generally applicable iodine-catalyzed
reaction of arylacetylenic acids and arylacetylenes with sodium sulfinates
provides arylacetylenic sulfones.
J. Meesin, P. Katrun, C. Pareseecharoen, M. Pohmakotr, V. Reutrakul, D.
Soorukram, C. Kuhakarn, J. Org. Chem.,
2016,
81, 2744-2752.

A metal-free direct condensation of sodium arylsulfinates and β,β-disubstituted
nitroalkenes provides allylic sulfones in excellent yields with a broad
substrate scope under mild conditions. The key step of this process was a Lewis
base-promoted equilibrium between nitroalkenes and allylic nitro compounds,
which contain more reactive C=C bonds toward sulfonyl radical addition.
X. Lei, L. Zheng, C. Zhang, X. Shi, Y. Chen, J. Org. Chem., 2018, 83,
1772-1778.

A copper-catalyzed amidation of allylic and benzylic C-H is applicable to the
coupling of a diverse set of hydrocarbon species with aryl, heteroaryl, and
alkyl sulfonamides and is tolerant of a variety of functional groups.
G. Pelletier, D. A. Powell, Org. Lett., 2006,
8, 6031-6034.

NaI-catalyzed direct condensation of sulfonamides and formamides enables N-sulfonyl
formamidine synthesis without hazardous reagents or transition-metal catalysts.
The green methodology features high atom economy, operational simplicity, and
good tolerance with diverse functional groups.
S. Chen, Y. Xu, X. Wan, Org. Lett., 2011,
13, 6152-6155.

A method for the synthesis of N-aroylated sulfoximines involves a
manganese oxide promoted C-H activation of methyl arenes to form an aroyl
intermediate which then reacts readily with N-chlorosulfoximines to form
a series of valuable aroyl sulfoximine derivatives in high yields.
D. L. Priebbenow, C. Bolm, Org. Lett., 2014,
16, 1650-1652.

Cu(OAc)2-promoted TBHP oxidative coupling reaction of formamides with thiophenols
gives S-aryl dialkyl thiocarbamates in high
yield under solvent-free conditions through direct C-H bond activation of formamides.
Y.-q. Yuan, S.-r. Guo, J.-n. Xiang, Synlett, 2013, 24,
443-448.

Tert-butyl hydroperoxide (TBHP) mediates a coupling of
sulfonylhydrazides with thiols in the presence of CuBr2 as catalyst
to afford thiosulfonates via a radical process.
G.-Y. Zhang, S.-S. Lv, A. Shoberu, J.-P. Zou, J. Org. Chem.,
2017, 82, 9801-9807.

An efficient phosphorylation of C(sp3)-H bonds of readily available
methyl arenes with diaryl phosphinic acids proceeds efficiently under
transition-metal-free reaction conditions via Bu4NI-catalyzed
dehydrogenative coupling to provide valuable organophosphorus compounds.
B. Xiong, G. Wang, C. Zhou, Y. Liu, P. Zhang, K. Tang, J. Org. Chem.,
2018, 83, 993-999.

A dual copper/photoredox-catalyzed approach for the construction of the
P(O)-N bond from commercially available aromatic amines and P(O)-H compounds
avoids toxic or corrosive reagents and does not require prefunctionalized
substrates. The reaction has a broad substrate scope and is suitable for the
synthesis of phosphonamides and phosphinamides.
K.-C. Yu, H. Li, Y.-H. Tu, H. Zhao, X.-G. Hu, Org. Lett., 2022, 24,
9130-9134.

A metal-free oxidative decarbonylative [3+2] annulation of terminal
alkynes with tertiary γ,δ-unsaturated aldehydes provides cyclopentenes, whereas
the reaction of terminal alkynes with 2-methyl-2-arylpropanals provides indenes.
The reactions offer broad substrate scope and excellent selectivity.
H.-X. Zou, Y. Li, X.-H. Yang, J. Xiang, J.-H. Li, J. Org. Chem., 2018, 83,
8581-8588.

A relay catalysis strategy enables a [3 + 1]-annulation reaction between
cyclopropane 1,1-diester and aromatic amines via lewis acid-catalyzed
nucleophilic ring opening of cyclopropane 1,1-diester with aromatic amine and (hypo)iodite-catalyzed
C-N bond formation. This reaction provides biologically important azetidines and
tetrahydroquinolines.
J.-Q. Han, H.-H. Zhang, P.-F. Xu, Y.-C. Luo, Org. Lett.,
2016, 18, 5212-5215.

A [2 + 1 + 1] radical tandem cycloaddition of styrenes, arylamines, and tert-butyl
hydroperoxide enables a regioselective synthesis of polysubstituted
1,2-oxazetidines. TBHP was employed in this conversion not only as the oxidant
but also as the oxygen source.
W. Liu, C. Chen, P. Zhou, H. Tan, Org. Lett.,
2017, 19, 5830-5832.

Palladium catalysis enables an unprecedented, highly effective synthesis of
γ-lactones from homoallylic alcohols in one step. The protocol affords aryl,
alkyl, and spiro γ-lactones directly from readily available homoallylic alcohols
in good yields with excellent functional group tolerance and high
chemoselectivity under mild conditions.
M. Zheng, P. Chen, L. Huang, W. Wu, H. Jiang, Org. Lett.,
2017, 19, 5756-5759.

Copper efficiently catalyzes a reaction of N-sulfonylaziridines and
N-alkylanilines in the presence of tert-butyl hydroperoxide to afford
functionalized imidazolidines via nucleophilic ring opening, sp3 C-H
functionalization, and C-N bond formation. The protocol can be used for the
enantiospecific synthesis of imidazolidines with excellent optical purity.
M. Sengoden, A. Bhowmick, T. Punniyamurthy, Org. Lett.,
2017, 19, 158-161.

Tetrabutylammonium iodide as the catalyst and tert-butyl hydroperoxide
in water (T-Hydro) as the oxidant enable a potential route for the construction
of functionalized oxazolidines and imidazolidines via oxidative C(sp3)-H
functionalization/C-O/C-N
bonds formations. The reaction is simple, regioselective, and effective at
moderate temperature with broad substrate scope.
V. Satheesh, M. Sengoden, T. Punniyamurthy, J. Org. Chem.,
2016, 81, 9792-9801.

A copper-catalyzed annulation of 1,3-dicarbonyl compound with diethylene glycol
gives 2,3-disubstituted furans in the presence of tert-butyl peroxide
(TBHP) via a sequential O- and C- functionalization of β-ketoester by diethylene
glycol. Diethylene glycol serves as a environmentally friendly and cheap
substitute of ethyne, that releases H2O and alcohol as clean wastes.
J.-T. Yu, B. Shi, H. Peng, S. Sun, H. Chu, Y. Jiang, J. Cheng, Org. Lett.,
2015,
17, 3643-3645.

A copper-mediated intermolecular annulation of alkyl ketones and β-nitrostyrenes
enables a regioselective synthesis of multisubstituted furan derivatives in good
yields.
M. Ghosh, S. Mishra, A. Hajra, J. Org. Chem.,
2015,
80, 5364-5368.

An iodine catalyzed C (sp3)-H functionalization of
tosylhydrazones with β-enamino esters under visible light irradiation
provides tri- and tetra-substituted pyrroles.
N. N. K. Reddy, D. Rawat, S. Adimurthy, J. Org. Chem., 2018, 83,
9412-9421.

An iron-catalyzed route for the regioselective synthesis of 1,3- and
1,3,5-substituted pyrazoles from the reaction of diarylhydrazones and vicinal
diols allows the conversions of a broad range of substrates.
N. Panda, A. K. Jena, J. Org. Chem., 2012,
77, 9401-9406.

A facile one-pot, transition-metal-free process enables the synthesis of various
polysubstituted oxazoles via t-BuOOH/I2-mediated domino
oxidative cyclization from readily available starting materials under mild
conditions.
H. Jiang, H. Huang, H. Cao, C. Qi, Org. Lett., 2010,
12, 5561-5563.

In a practical and simple synthesis of 2,5-disubstituted oxazoles via an
iodine-catalyzed tandem oxidative cyclization, a wide range of common commercial
aromatic aldehydes can be used as reaction substrates, which displayed excellent
functional group compatibility.
C. Wan, L. Gao, Q. Wang, J. Zhang, Z. Wang, Org. Lett., 2010,
12, 3902-9305.

I2-catalyzed C-O bond formation and dehydrogenation with TBHP enables
a general method for the synthesis of oxazolines and oxazoles from β-acylamino
ketones. Depending on the base, either oxazolines or oxazoles were selectively
produced.
W.-C. Gao, F. Hu, Y.-M. Huo, H.-H. Chang, X. Li, W.-L. Wei, Org. Lett.,
2015,
17, 3914-3917.

I2-catalyzed C-O bond formation and dehydrogenation with TBHP enables
a general method for the synthesis of oxazolines and oxazoles from β-acylamino
ketones. Depending on the base, either oxazolines or oxazoles were selectively
produced.
W.-C. Gao, F. Hu, Y.-M. Huo, H.-H. Chang, X. Li, W.-L. Wei, Org. Lett.,
2015,
17, 3914-3917.

A highly efficient copper-catalyzed tandem oxidative cyclization gives
polysubstituted oxazoles from readily available starting materials under mild
conditions. This is an attractive alternative method for the synthesis of
oxazole derivatives.
C. Wang, J. Zhang, S. Wang, J. Fan, Z. Wang, Org. Lett., 2010,
12, 2338-2341.

An I2-catalyzed oxidative cross coupling of N-sulfonyl
hydrazones with isocyanides in the presence of TBHP as terminal oxidant enables
the synthesis of 5-aminopyrazoles through formal [4 + 1] annulation via in situ
azoalkene formation. Notable features are a metal/alkyne-free strategy, atom
economy, catalytic I2, broad functional group tolerance, good
reaction yields and short time.
G. C. Senadi, W.-P. Hu, T.-Y. Lu, A. M. Garkhedkar, J. K. Vandavasi, J.-J.
Wang, Org. Lett.,
2015,
17, 1521-1524.

A general and metal-free synthesis of 1,3,5-trisubstituted 1,2,4-triazoles from
hydrazones and aliphatic amines has been achieved under oxidative conditions via
a cascade C-H functionalization, double C-N bonds formation, and oxidative
aromatization sequence in the presence of iodine as catalyst.
Z. Chen, H. Li, W. Dong, M. Miao, H. Ren, Org. Lett., 2016, 18,
1334-1337.

A iodine-catalyzed tandem Michael addition/oxidative annulation of allenes and
enamines provides polysubstituted pyrroles in an efficient and highly
regioselective way in good yields under mild conditions.
Y. Wang, C.-M. Jiang, H.-L. Li, F.-S. He, X. Luo, W.-P. Deng, J. Org. Chem.,
2016, 81, 8653-8658.

A TBAI/TBHP catalyst system enables a facile synthetis of highly functionalized
3-aminothiophenes from readily available allenes and thioamides in very good
yields under mild reaction conditions via a tandem thio-Michael addition,
oxidative annulation, and 1,2-sulfur migration pathway.
T. Han, Y. Wang, H.-L. Li, X. Luo, W.-P. Deng, J. Org. Chem.,
2018, 83, 1538-1542.

A Brřnsted acid accelerated oxidative radical annulation of sulfonyl
hydrazones with simple olefins provides six-membered
heterocycles. The method offers a rapid and efficient approach to tetrahydropyridazines
which are key structural motifs in pharmaceutically active compounds.
X. Zhong, J. Lv, S. Luo, Org. Lett.,
2016, 18, 3150-3153.

D-Glucose can be used as an efficient C1 synthon in the synthesis of
benzimidazoles from o-phenylenediamines via an oxidative cyclization
strategy. This method offers broad functional group tolerance, a biorenewable
methine source, excellent reaction yields, a short reaction time, and water as
an environmentally benign solvent.
D. Raja, A. Philips, P. Palani, W.-Y. Lin, S. Devikala, G. C. Senadi, J. Org. Chem., 2020, 85,
11531-11540.

A one-pot, multicomponent reaction enables the transformation of commercial aryl
amines, aldehydes, and azides into valuable benzimidazole structural units with
wide substrate scope and diversity via an efficient copper-catalyzed amination
of N-aryl imines, in which imine acts as a directing group by chelating
to the metal center.
D. Mahesh, P. Sadhu, T. Punniyamurthy, J. Org. Chem.,
2015,
80, 1644-1650.

A copper(II)-catalyzed oxidative cross-coupling of anilines, primary alkyl
amines, and sodium azide provides benzimidazoles in the presence of TBHP at
moderate temperature via a domino C-H functionalization, transimination,
ortho-selective amination, and a cyclization sequence. The reaction offers
broad substrate scope and functional group compatibility.
D. Mahesh, P. Sadhu, T. Punniyamurthy, J. Org. Chem.,
2016,
81, 3227-3234.

A selective, TBHP/KI-promoted C-O bond cleavage of ethers followed by
annulation with anilines and elemental sulfur provides a wide range of 2-aryl-,
2-heteroaryl-, and 2-alkyl-substituted benzothiazoles with satisfactory yields and good functional group compatibility under
transition-metal-free conditions.
J. Zhang, X. Zhao, P. Liu, P. Sun, J. Org. Chem., 2019, 84,
12596-12605.

I2 and TBHP mediate a convenient synthesis of 2-acylbenzothiazoles in
very good yields from acetophenones and benzothiazoles.
The formal acylation of the benzothiazoles is achieved through a sequence involving
formation of an aryl glyoxal, ring-opening of the
benzothiazole followed by condensation of the amino group with the aryl glyoxal,
cyclization and oxidation.
B. Wang, Q. Zhang, Z. Guo, K. Ablajan, Synthesis, 2020, 52,
3058-3064.

Recombination of alkyl radicals derived from indolin-2-ones with the tert-butylhydroperoxy
radical affords 3-(tert-butylperoxy)indolin-2-one intermediates that can
be further transformed into indoline-2,3-diones under air. This strategy
provides a simple, effcient, and metal-free route to indolinediones.
W.-W. Ying, W.-M. Zhu, H. Liang, W.-T. Wei,
Synlett, 2018, 29, 215-218.

The use of a catalytic amount I2 and TBHP as stoichiometric oxidant
enables a simple and atom economic one-pot synthesis of isatins from 2′-aminoacetophenones
via oxidative amido cyclization of the sp3 C-H bond. In the presence
of a stoichiometric amount of iodine, the reaction yields iodoisatines. The
reaction proceeds through sequential iodination, Kornblum oxidation, and
amidation in one pot.
A. Ilangovan, G. Satish, J. Org. Chem., 2014,
79, 4984-4991.

An efficient oxo-sulfonylation protocol enables the synthesis of N-sulfonylated
indazolones from 2H-indazoles employing sulfinic acid as a sulfonylating
agent in the presence of tert-butyl hydroperoxide (TBHP) under ambient
air. A series of structurally diverse 1-sulfonylindazol-3(2H)-one
derivatives were obtained in good yields.
P. Ghosh, S. Mondal, A. Hajra,
Org. Lett., 2020, 22, 1086-1090.

A metal-free sequential dual oxidative amination of C(sp3)-H bonds
under ambient conditions affords imidazo[1,5-a]pyridines in very good yields.
The reaction involves two oxidative C-N couplings and one oxidative
dehydrogenation process with six hydrogen atoms removed.
Y. Yan, Y. Zhang, Z. Zha, Z. Wang, Org. Lett., 2013,
15, 2274-2277.

An efficient transition-metal-free oxidative cyclization reaction of alkynes
with isatins enables a facile synthesis of structurally diverse 4-quinolones.
S.-F. Jiang, C. Xu, Z.-W. Zhou, Q. Zhang, X.-H. Wen, F.-C. Jia, A.-X. Wu, Org. Lett.,
2018, 20, 4231-4234.

A simple switch in reaction conditions enables facile and efficient syntheses
of various valuable 3-aryl- and 3-aroylcoumarins by direct arylation and
aroylation of coumarins with glyoxals in a metal-free manner. This approach
accommodates a broad substrate scope and high yields of the two types of
cross-coupling reactions starting from identical starting materials.
A. Moazzam, M. Khodadadi, F. Jafarpour, M. Ghandi, J. Org. Chem., 2022, 87,
3630-3637.

A facile approach allows the synthesis of 2-phenylquinazolines via a tandem
reaction following sp3 C-H functionalization. Twenty-five examples of
2-phenylquinazolines were obtained from easily available 2-aminobenzophenones
and benzylic amines with good to excellent yields.
J. Zhang, D. Zhu, C. Yu, C. Wan, Z. Wang, Org. Lett., 2010,
12, 2841-2843.

A facile and efficient method for the synthesis of 2-phenylquinazolines from
2-aminobenzophenones and benzylamines us catalyzed by ceric ammonium nitrate (CAN)-TBHP
in acetonitrile. The corresponding 2-phenylquinazolines were obtained in good to
excellent yields.
K. Karnakar, J. Shangkar, S. N. Murthy, K. Ramesch, Y. V. D. Nageshwar, Synlett, 2011,
1089-1096.

A synergetic tert-butyl hydroperoxide/K3PO4-promoted
oxidative cyclization enables a facile synthesis of various functionalized
quinazolin-4(3H)-ones from commercially available isatins and amidine
hydrochlorides at room temperature.
F.-C. Jia, Z.-W. Zhou, C. Xu, Y.-D. Wu, A.-X. Wu, Org. Lett.,
2016, 18, 2942-2945.

An efficient and facile reaction of quinazoline-3-oxides with primary amines
provides a broad range of quinazolin-4(3H)-ones under metal-free and mild
reaction conditions employing tert-butyl hydroperoxide as the oxidant.
Remarkably, a precursor for the synthesis of bioactive evodiamine and rutaempine
was conveniently obtained in good yield.
J. Luo, J. Wan, L. Wu, L. Yang, T. Wang, J. Org. Chem., 2022, 87,
9864-9874.

A facile metal-free oxidative amination of benzoxazole by activation of C-H
bonds with secondary or primary amines in the presence of catalytic iodine in
aqueous tert-butyl hydroperoxide proceeds smoothly at ambient temperature
under neat reaction condition to furnish products in high yields. This
user-friendly method produces only tertiary butanol and water as byproducts.
M. Lamani, K. R. Prabhu, J. Org. Chem., 2011,
76, 7938-7944.

Catalytic amounts of tetrabutylammoniumiodide (TBAI), aqueous solutions of H2O2
or TBHP as co-oxidant enabled an efficient transition-metal-free amination of
benzoxazoles under mild reaction conditions, to yield highly desirable
2-aminobenzoxazoles in good yields. First mechanistic experiments indicate the
in situ iodination of the secondary amine as the putative mode of activation.
T. Froehr, C. P. Sindlinger, U. Kloeckner, P. Finkbeiner, B. J. Nachtsheim, Org. Lett., 2011,
13, 3754-3757.

A metal-free cross-dehydrogenative coupling between quinoxalinones and amines
in the presence of catalytic iodine and aqueous tert-butyl
hydroperoxide as the terminal oxidant provides 3-aminoquinoxalinones in good yields in dioxane as solvent. The reaction is highly versatile and
exhibits good functional group tolerance with a range of primary and secondary
amines.
A. Gupta, M. S. Deshmuk, N. Jain, J. Org. Chem.,
2017, 82, 4748-4792.

Cu-catalyzed sp3 C-H bond activation α to the nitrogen atom of o-alkynylated
N,N-dimethylamines followed by an intramolecular nucleophilic attack with
the alkyne, using an aqueous solution of tert-butyl hydroperoxide (TBHP)
as the oxidant, enables a tandem catalytic synthesis of 3-aroylindoles. In this
synthesis, both C-C and C-O bonds are installed at the expense of two sp3
C-H bond cleavages.
A. Gogoi, S. Guin, S. K. Rout, B. K. Patel, Org. Lett., 2013,
15, 1802-1805.

An I2/TBHP-mediated oxidation of commercially available indoles
affords isatins in moderate to good yields.
Y. Zi, Z.-J. Cai, S.-Y. Wang, S.-J. Ji, Org. Lett., 2014,
16, 3094-3097.

A copper/acid-catalyzed oxidative carbamoylation of various
electron-deficient nitrogen heteroarenes (isoquinolines, quinolines, pyridines,
phenanthridines, quinoxalines, and phthalazines) with hydrazinecarboxamide
hydrochlorides provides structurally diverse nitrogen-heteroaryl carboxamides as
single regioisomers in moderate to excellent yields.
Z.-Y. He, C.-F. Huang, S.-K. Tian, Org. Lett.,
2017, 19, 4850-4853.

An iron-catalyzed C(sp3)-H oxidation, intramolecular C-N bond
formation, and aromatization enables an efficient synthesis of quinazolines from
2-alkylamino N-H ketimine derivatives, which can be prepared by addition of
various organometallic reagents to 2-alkylamino benzonitriles.
C.-y. Chen, F. He, G. Tang, H. Yuan, N. Li, J. Wang, R. Faessler, J. Org. Chem., 2018, 83,
2395-2401.

A Cu-catalyzed denitrogenative transannulation of 3-aminoindazoles provides
diverse functionalized 3-aminobenzothiophenes and 1-aminoisoquinolines. This
transformation proceeds via an "extrude-and-sew" strategy with an unprecedented
radical reactivity of 3-aminoindazoles.
Y. Zhou, Y. Wang, Y. Lou, Q. Song,
Org. Lett., 2019, 21, 8869-8873.

An n-Bu4NI-catalyzed reaction of 3-methylindoles with
primary amines using TBHP as the unique oxidant provides broad range of
quinazolinones in very good yields. The reaction involves oxygenation,
nitrogenation, ring-opening, and recyclization.
J. He, J. Dong, L. Su, S. Wu, L. Liu, S.-F. Yin, Y. Zhou,
Org. Lett., 2020, 22, 2522-2526.

An annulation of 2-cyanoaryl acrylamides via C=C double bond cleavage enables
a facile and efficient synthesis of functionalized 4-amino-2-quinolones, which
are important N-heterocycles. In this transformation, a THF radical plays
a crucial role.
W.-J. Xia, T.-G. Fan, Z.-W. Zhao, X. Chen, X.-X. Wang, Y.-M. Li, Org. Lett., 2021, 23,
6158-6163.

Reactions between readily available o-hydroxyphenyl enaminones and
various alkenes enable a a step economical and general synthesis
of 3-vinyl chromones.
L. Fu, Z. Xu, J.-P. Wan, Y. Liu, Org. Lett., 2020, 22, 9518-9523.

The use of the Langlois reagent enables an efficient and regioselective synthesis of various 3-(trifluoromethyl)chromones
from enamino ketones. This convenient procedure offers
high product purity and yield.
P. Thota, K. Sheelam, S. Kottawar, K. Shivakumar, M. Kaliyaperumal, S. Yennam,
M. Behera, Synlett, 2022,
33,
1660-1664.

tert-Butyl hydroperoxide promotes an oxidative annulation reaction of
isatins with 2-(trimethylsilyl)aryl triflates for the convenient synthesis of
acridone derivatives. The reaction may proceed via consecutive
Baeyer-Villiger-type rearrangement followed by an intermolecular cyclization.
This convenient method offers broad substrate scope and good functional group
tolerance.
M. Luo, N. Dong, M. Zhu, Y. Wang, C. Xu, G. Yin, J. Org. Chem., 2023, 88,
9419-9423.

Aqueous tert-butyl hydroperoxide (70%) is an inexpensive reagent for the
regioselective and chemoselective deprotection of terminal acetonide groups.
Various acetonide derivatives furnish the corresponding deprotected diols in
good yields, while a large number of acid labile protecting functional groups
and other functional moieties were found to be unaffected under the conditions.
M. R. Maddani, K. R. Prabhu, Synlett, 2011,
821-825.

A mild and practical protocol for the copper-mediated trifluoromethylation of
aryl and heteroaryl boronic acids using NaSO2CF3
(Langlois’ reagent) and TBHP proceeds at room temperature under ambient
conditions. The products can be readily purified by extraction or column
chromatography.
Y. Ye, S. A. Künzi, M. S. Sanford, Org. Lett., 2012,
14, 4979-7981.

A copper-catalyzed decarboxylative trifluoromethylation of various
α,β-unsaturated carboxylic acids was achieved by using a stable and inexpensive
solid, sodium trifluoromethanesulfinate (CF3SO2Na,
Langlois reagent). In addition, an iron-catalysis enables a difluoromethylation
of aryl-substituted acrylic acids by using zinc difluoromethanesulfinate (DFMS,
(CF2HSO2)2Zn, Baran reagent) via a similar
radical process.
Z. Li, Z. Cui, Z.-Q. Liu, Org. Lett., 2013,
15, 406-409.

Halosulfonylation of terminal alkynes was achieved with sulfonylhydrazides as
the sulfonyl precursor and inexpensive iron halide as halide source in the
presence of TBHP to yield (E)-β-chloro and bromo vinylsulfones regio- and
stereoselectively.
X. Li, X. Shi, M. Fang, X. Xu, J. Org. Chem., 2013,
78, 9499-9504.

A TBHP/TBAI-mediated reaction of propargyl alcohols with sulfonyl hydrazides in
the presence of HOAc provides allenyl sulfones in good yields in a short
reaction time via HOAc-promoted sulfonohydrazide intermediate formation,
sequential C-O, C-N, and N-S bond cleavage, and C-S bond formation. This
reaction shows highly functional group compatibility and excellent
regioselectivity.
Z. Yang, W.-J. Hao, S.-L. Wang, J.-P. Zhang, B. Jiang, G. Li, S.-J. Tu, J. Org. Chem.,
2015,
80, 9224-9230.

