Further Information
Literature
Related Reactions
Dakin Reaction
Baeyer-Villiger Oxidation

The Baeyer-Villiger Oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts ketones to esters and cyclic ketones to lactones. The Baeyer-Villiger can be carried out with peracids, such as MCBPA, or with hydrogen peroxide and a Lewis acid.
The regiospecificity of the reaction depends on the relative migratory ability of the substituents attached to the carbonyl. Substituents which are able to stabilize a positive charge migrate more readily, so that the order of preference is: tert. alkyl > cyclohexyl > sec. alkyl > phenyl > prim. alkyl > CH3. In some cases, stereoelectronic or ring strain factors also affect the regiochemical outcome.
Mechanism of the Baeyer-Villiger Oxidation



Recent Literature

Baeyer-Villiger Oxidation of Ketones to Esters with Sodium Percarbonate/Trifluoroacetic
Acid
G. A. Olah, Q. Wang, N. J. Trivedi, G. K. S. Prakash, Synthesis,
1991, 739-740.

Selenoxides as Catalysts for Epoxidation and Baeyer-Villiger Oxidation with
Hydrogen Peroxide
M. A. Goodman, M. R. Detty, Synlett,
2006, 1100-1104.
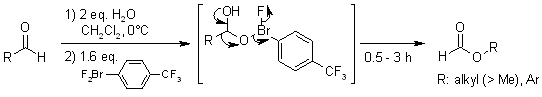
Hypervalent λ3-Bromane Strategy for Baeyer-Villiger Oxidation:
Selective Transformation of Primary Aliphatic and Aromatic Aldehydes to
Formates, Which is Missing in the Classical Baeyer-Villiger Oxidation
Y. Yoshida, K. Murakami, H. Yorimitsu, K. Oshima, J. Am. Chem. Soc., 2010,
132, 9236-9239.

General Metal-Free Baeyer-Villiger-Type Synthesis of Vinyl Acetates
B. Poladura, A. Martínez-Castañeda, H. Rodríguez-Solla, R. Llavona, C. Concellón,
V. del Amo, Org. Lett., 2013,
15, 2810-2813.

Green Oxidation of Ketones to Lactones with Oxone in Water
V. Bertolini, R. Appiani, M. Pallavicini, C. Bolchi, J. Org. Chem., 2021, 86,
15712-15716.

The Baeyer-Villiger oxidation of ketones with bis(trimethylsilyl) peroxide
in the presence of ionic liquids as the solvent and catalyst
S. Baj, A. Chrobok, R. Słupska, Green Chem., 2009,
11, 279-282.

Enantioselective Baeyer-Villiger Oxidation: Desymmetrization of Meso Cyclic
Ketones and Kinetic Resolution of Racemic 2-Arylcyclohexanones
L. Zhou, X. H. Liu, J. Ji, Y. H. Zhang, X. L. Hu, L. L. Lin, X. M. Feng, J. Am. Chem. Soc., 2012,
134, 17023-17026.

Asymmetric Baeyer-Villiger Reaction with Hydrogen Peroxide Catalyzed by a
Novel Planar-Chiral Bisflavin
S. Murahashi, S. Ono, Y. Imada,
Angew. Chem. Int. Ed., 2002, 41,
2366-2368.

Stereospecific Nitrogen Insertion Using Amino Diphenylphosphinates: An
Aza-Baeyer-Villiger Rearrangement
M. Ong, M. Arnold, A. W. Walz, J. M. Wahl, Org. Lett.,
2022, 24, 6171-6175.

Conversion of Cyclic Acetals to Hydroxy Esters by MCPBA Oxidation
J. Y. Kim, H. Rhee, M. Kim, J. Korean Chem. Soc., 2002, 46,
479-483.

Transition-Metal-Free Approach to Acridone Derivatives by TBHP-Promoted
Oxidative Annulation of Isatins with Arynes
M. Luo, N. Dong, M. Zhu, Y. Wang, C. Xu, G. Yin, J. Org. Chem., 2023, 88,
9419-9423.
