Further Information
Literature
Related Reactions
Appel Reaction
Staudinger Reaction
Synthesis of esters
Mitsunobu Reaction


The Mitsunobu Reaction allows the conversion of primary and secondary alcohols to esters, phenyl ethers, thioethers and various other compounds. The nucleophile employed should be acidic, since one of the reagents (DEAD, diethylazodicarboxylate) must be protonated during the course of the reaction to prevent from side reactions.
Suitable nitrogen nucleophiles include phthalimide or hydrogen azide; subsequent hydrolysis (in the case of using phthalimide, see Gabriel Synthesis) or selective reduction (in the case of azide formation, see Staudinger Reaction) makes the corresponding amines accessible.
Mechanism of the Mitsunobu Reaction
The triphenylphosphine combines with DEAD to generate a phosphonium intermediate that binds to the alcohol oxygen, activating it as a leaving group. Substitution by the carboxylate, mercaptyl, or other nucleophile completes the process.

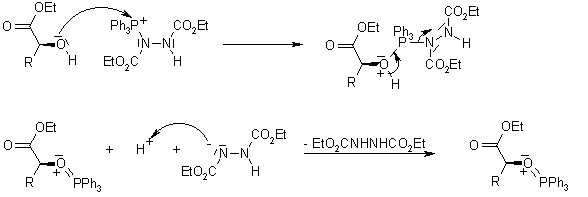
The reaction proceeds with clean inversion, which makes the Mitsunobu Reaction with secondary alcohols a powerful method for the inversion of stereogenic centers in natural product synthesis.

Side Reaction:

New protocols have been developed which allow better removal of side products and/or the conversion of more basic nucleophiles.
Recent Literature

Mitsunobu Reaction with 4-(Diphenylphosphino)benzoic Acid: A
Separation-Friendly Bifunctional Reagent that Serves as Both a Reductant and a
Pronucleophile
N. Muramoto, K. Yoshino, T. Misaki, T. Sugimura, Synthesis, 2013, 45,
931-935.

Easily Prepared Azopyridines As Potent and Recyclable Reagents for Facile
Esterification Reactions. An Efficient Modified Mitsunobu Reaction
N. Iranpoor, H. Firouzabadi, D. Khalili, S. Motevalli, J. Org. Chem., 2008,
73, 4882-4887.

Systematic Evaluation of 2-Arylazocarboxylates and 2-Arylazocarboxamides as
Mitsunobu Reagents
D. Hirose, M. Gazvoda, J. Košmrlj, T. Taniguchi, J. Org. Chem., 2018, 83,
4714-4729.

Simplification of the Mitsunobu Reaction. Di-p-chlorobenzyl Azodicarboxylate:
A New Azodicarboxylate
B. H. Lipshutz, D. W. Chung, B. Rich, R. Corral, Org. Lett., 2006,
8, 5069-5072.

N-Methyl Allylic Amines from Allylic Alcohols by Mitsunobu Substitution
Using N-Boc Ethyl Oxamate
B. C. van Veen, S. M. Wales, J. Clayden, J. Org. Chem., 2021, 86,
8538-8543.

A convenient Two-Step Procedure for the Synthesis of Substituted Allylic Amines
from Allylic Alcohols
S. E. Sen, S. L. Roach, Synthesis, 1995, 756-758.

Mitsunobu Approach to the Synthesis of Optically Active α,α-Disubstituted
Amino Acids
J. E. Green, D. M. Bender, S. Jackson, M. J. O'Donnell, J. R. McCarthy, Org. Lett., 2009,
11, 807-810.

One-Carbon Homologation of Primary Alcohols to Carboxylic Acids, Esters, and
Amides via Mitsunobu Reactions with MAC Reagents
N. Kagawa, A. E. Nibbs, V. H. Rawal, Org. Lett.,
2016, 18, 2363-2366.

Fluorous Mitsunobu reagents and reactions
S. Dandapani, D. P. Curran, Tetrahedron,
2002, 58, 3855-3864.

Use of Sonication for the Coupling of Sterically Hindered Substrates in the
Phenolic Mitsunobu Reaction
S. D. Lepore, Y. He,
J. Org. Chem., 2003, 68, 8261-8263.

Organocatalytic Mitsunobu Reactions
T. Y. S. But, P. H. Toy, J. Am. Chem. Soc., 2006,
128, 9636-9637.

Stereoselective Synthesis of Styrene Oxides via a Mitsunobu Cyclodehydration
S. A. Weissman, K. Rossen, P. J. Reider,
Org. Lett., 2001, 3, 2513-2513.

Carbon Nucleophiles in the Mitsunobu Reaction. Mono and Dialkylation of
Bis(2,2,2-trifluorethyl) Malonates
J. M. Takacs, Z. Xu, X.-T. Jiang, A. P. Leonov, G. C. Theriot, Org. Lett., 2002, 4, 3843-3845.

O-TBS-N-tosylhydroxylamine: A Reagent for Facile Conversion of
Alcohols to Oximes
K. Kitahara, T. Toma, J. Shimokawa, T. Fukuyama, Org. Lett., 2008,
10, 2259-2261.

An Efficient, One-Pot Synthesis of S-Alkyl Thiocarbamates from the
Corresponding Thiols Using the Mitsunobu Reagent
D. Chaturvedi, N. Mishra, V. Mishra, Synthesis, 2008,
355-357.

Synthesis of N-H vinylaziridines: a comparative study
B. Olofsson, R. Wijtmans, P. Somfai, Tetrahedron, 2002, 58, 5979-5982.

New Synthesis of 1,1-Substituted Hydrazines by Alkylation of N-Acyl-
or N-alkyloxycarbonylaminophthalimide Using the Mitsunobu Protocol
N. Brosse, M.-F. Pinto, B. Jamart-Grégoire, J. Org. Chem., 2000,
65, 4370-4374.

Exploration of the Mitsunobu Reaction with Tosyl- and Boc-Hydrazones as
Nucleophilic Agents
J. M. Keith, L. Gomez, J. Org. Chem., 2006, 71, 7113-7116.

Single-Step Process for the Reductive Deoxygenation of Unhindered Alcohols
A. G. Myers, M. Movassaghi, B. Zheng, J. Am. Chem. Soc.,
1997, 119, 8572-8573.

Conversion of Alcohols, Thiols, Carboxylic Acids, Trimethylsilyl Ethers, and
Carboxylates to Thiocyanates with Triphenylphosphine/Diethylazodicarboxylate/NH4SCN
N. Iranpoor, H. Firouzabadi, B. Akhlaghinia, R. Azadi, Synthesis, 2004,
92-96.

Successive Nucleophilic and Electrophilic Allylation for the Catalytic
Enantioselective Synthesis of 2,4-Disubstituted Pyrrolidines
G. Luo, M. Xiang, M. J. Krische,
Org. Lett., 2019, 21, 2493-2497.

A Modular Synthesis of 2-Alkyl- and 2-Arylchromans via a Three-Step Sequence
R. K. Orr, L.-C. Campeau, H. R. Chobanian, H. M. McCabe Dunn, B. Pio, C. W.
Plummer, A. Nolting, R. T. Ruck, Synthesis, 2017,
49, 657-666.
