Further Information
Literature
Related Reactions
Cadiot-Chodkiewicz Coupling
Stille Coupling
Synthesis of substituted Alkynes
Synthesis of 1,3-Enynes
Sonogashira Coupling

This coupling of terminal alkynes with aryl or vinyl halides is performed with a palladium catalyst, a copper(I) cocatalyst, and an amine base. Typically, the reaction requires anhydrous and anaerobic conditions, but newer procedures have been developed where these restrictions are not important.
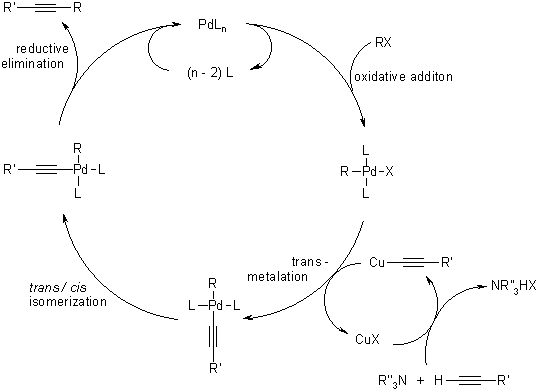
Mechanism of the Sonogashira Coupling
Recent Literature

The First Applications of Carbene Ligands in Cross-Couplings of Alkyl
Electrophiles: Sonogashira Reactions of Unactivated Alkyl Bromides and Iodides
M. Eckhardt, G. C. Fu, J. Am. Chem. Soc., 2003, 125, 13642-13643.

Efficient Sonogashira Coupling Reaction Catalyzed by Palladium(II)
β-Oxoiminatophosphane Complexes under Mild Conditions
D.-H. Lee, H. Qiu, M.-H. Cho, I.-M. Lee, M.-J. Jin, Synlett, 2008,
1657-1660.

A Convenient Procedure for Sonogashira Reactions Using Propyne
J. L. Alterman, G. A. Kraus, Synthesis, 2022, 54,
655-657.

Hydrazone-Promoted Sonogashira Coupling Reaction with Aryl Bromides at Low
Palladium Loadings
T. Mino, S. Suzuki, K. Hirai, M. Sakamoto, T. Fujita, Synlett, 2011,
1277-1280.

Sonogashira Couplings Catalyzed by Collaborative (N-Heterocyclic
Carbene)-Copper and -Palladium Complexes
C. W. D. Gallop, M.-T. Chen, O. Navarro, Org. Lett., 2014,
16, 3724-3727.

Sustainable HandaPhos-ppm Palladium Technology for Copper-Free
Sonogashira Couplings in Water under Mild Conditions
S. Handa, J. D. Smith, Y. Zhang, B. S. Takale, F. Gallou, B. H. Lipshutz, Org. Lett.,
2018, 20, 542-545.

Designing Homogeneous Copper-Free Sonogashira Reaction through a Prism of
Pd-Pd Transmetalation
B. A. Martek, M. Gazvoda, D. Urankar, J. Komrlj,
Org. Lett., 2020, 22, 4938-4943.

Pd(PhCN)2Cl2/P(t-Bu)3: A Versatile
Catalyst for Sonogashira Reactions of Aryl Bromides at Room Temperature
T. Hundertmark, A. F. Littke, S. L. Buchwald, G. C. Fu,
Org. Lett., 2000, 2, 1729-1731.

Dimethylisosorbide (DMI) as a Bio-Derived Solvent for Pd-Catalyzed
Cross-Coupling Reactions
K. L. Wilson, J. Murray, H. F. Sneddon, C. Jamieson, A. J. B. Watson, Synlett, 2018, 29,
2293-2297.

lycosyl Triazole Ligand for Temperature-Dependent Competitive Reactions of
Cu-Catalyzed Sonogashira Coupling and Glaser Coupling
N. Mishra, S. K. Singh, A. S. Singh, A. K Agrahari, V. K. Tiwari, J. Org. Chem., 2021, 86,
17890-17895.

Gold and Palladium Combined for the Sonogashira Coupling of Aryl and
Heteroaryl Halides
B. Panda, T. K. Sarkar, Synthesis, 2013, 45,
817-829.

Modified Palladium-Catalyzed Sonogashira Cross-Coupling Reactions under
Copper-, Amine-, and Solvent-Free Conditions
Y. Liang, Y.-X. Xie, J.-H. Li, J. Org. Chem., 2006, 71, 379-381.

Application of recoverable nanosized palladium(0) catalyst in Sonogashira
reaction
P. Li, L. Wang, H. Li, Tetrahedron, 2005,
61, 8633-8640.

Sonogashira Couplings of Aryl Bromides: Room Temperature, Water Only, No
Copper
B. H. Lipshutz, D. W. Chung, B. Rich, Org. Lett.,
2008,
10, 3793-3796.

Copper- and Ligand-Free Sonogashira Reaction Catalyzed by Pd(0)
Nanoparticles at Ambient Conditions under Ultrasound Irradiation
A. R. Gholap, K. Venkatesan, R. Pasricha, T. Daniel, R. J. Lahoti, K. V.
Srinivasan, J. Org. Chem., 2005, 70, 4869-4872.

CsF-Mediated in Situ Desilylation of TMS-Alkynes for Sonogashira Reaction
J. S. Capani Jr., J. E. Cochran, J. Liang, J. Org. Chem., 2019, 84,
9378-9384.

Palladacycle-Catalyzed Deacetonative Sonogashira Coupling of Aryl Propargyl
Alcohols with Aryl Chlorides
H. Hu, F. Yang, Y. Wu, J. Org. Chem., 2013,
78, 10506-10507.

Efficient Copper-Free PdCl2(PCy3)2-Catalyzed Sonogashira Coupling of Aryl
Chlorides with Terminal Alkynes
C. Yi, R. Hua, J. Org. Chem., 2006, 71, 2535-2537.

Efficient Palladium-Catalyzed Coupling of Aryl Chlorides and Tosylates with
Terminal Alkynes: Use of a Copper Cocatalyst Inhibits the Reaction
D. Gelman, S. L. Buchwald, Angew. Chem. Int. Ed., 2003, 42,
5993-5996.

Rapid and Efficient Pd-Catalyzed Sonogashira Coupling of Aryl Chlorides
H. Huang, H. Liu, H. Jiang, K. Chen, J. Org. Chem., 2008,
73, 6037-6040.

A highly efficient Pd-catalyzed Sonogashira coupling of terminal alkynes with both unreactive
electron-rich fluoroarenes and electron-poor fluoroarenes afforded the corresponding internal
alkynes in good yields in the presence of LiHMDS.
J. He, K. Yang, J. Zhao, S. Cao,
Org. Lett., 2019, 21, 9714-9718.

Copper-Free Sonogashira Coupling Reaction with PdCl2
in Water under Aerobic Conditions
B. Liang, M. Dai, J. Chen, Z. Yang, J. Org. Chem., 2005, 70, 391-393.

Sonogashira Coupling Reaction with Diminished Homocoupling
A. Elangovan, Y.-H. Wang, T.-I. Ho, Org. Lett.,
2003, 5, 1841-1844.

Carbamoyl-Substituted N-Heterocyclic Carbene Complexes of
Palladium(II): Application to Sonogashira Cross-Coupling Reactions
R. A. Batey, M. Shen, A. J. Lough, Org. Lett., 2002, 1411-1414.

Rapid Homogeneous-Phase Sonogashira Coupling Reactions Using Controlled
Microwave Heating
M. Erdélyi, A. Gogoll, J. Org. Chem., 2001,
66, 4165-4169.

One-Pot Procedure for the Synthesis of Unsymmetrical Diarylalkynes
R. Severin, J. Reimer, S. Doye, J. Org. Chem., 2010,
75, 3518-3521.

Diastereodivergent Reductive Cross Coupling of Alkynes through Tandem
Catalysis: Z- and E-Selective Hydroarylation of Terminal Alkynes
M. K. Armstrong, M. B. Goodstein, G. Lalic, J. Am. Chem. Soc.,
2018,
140, 10233-10241.

One-Pot Synthesis of Diarylalkynes Using Palladium-Catalyzed Sonogashira Reaction and Decarboxylative Coupling of sp Carbon and sp2 Carbon
J. Moon, M. Jeong, H. Nam, J. Ju, J. H. Moon, H. M. Jung, S. Lee, Org. Lett., 2008,
10, 945-948.

Decarbonylative Sonogashira Cross-Coupling of Carboxylic Acids
C. Liu, M. Szostak, Org. Lett., 2021, 23,
4726-4730.

Investigation of an Efficient Palladium-Catalyzed C(sp)-C(sp) Cross-Coupling
Reaction Using Phosphine-Olefin Ligand: Application and Mechanistic Aspects
W. Shi, Y. Luo, X. Luo, L. Chao, H. Zhang, J. Wang, A. Lei, J. Am. Chem. Soc., 2008,
130, 14713-14720.

Sonogashira Cross-Coupling of Aryl Ammonium Salts by Selective C-N
Activation Catalyzed by Air- and Moisture-Stable, Highly Active [Pd(NHC)(3-CF3-An)Cl2]
(An = Aniline) Precatalysts
P. Lei, Y. Wang, C. Zhang, X. Hu, J. Feng, Z. Ma, X. Liu, R. Szostak, M.
Szostak, Org. Lett.,
2022, 24, 6310-6315.

A Convenient, Efficient, and Inexpensive Copper(I) Complex Catalyzed
Sonogashira Cross-Coupling of o-Iodoanilines with Terminal Alkynes
X. Chen, X.-Y. Zhou, Synthesis, 2023,
55, 1213-1220.

Pd/Cu-Catalyzed Vinylation of Terminal Alkynes with
(2-Bromoethyl)diphenylsulfonium Triflate
X.-X. Ming, S. Wu, Z.-Y. Tian, J.-W. Song, C.-P. Zhang, Org. Lett., 2021, 23,
6795-6800.

Discovery of A Novel Palladium Catalyst for the Preparation of Enynes with a
Copper- and Ligand-Free Sonogashira Reaction
X. Mi, M. Huang, Y. Feng, Y. Wu, Synlett, 2012, 23,
1257-1261.

Sonogashira Reaction Using Arylsulfonium Salts as Cross-Coupling Partners
Z.-Y. Tian, S.M. Wang, S.-J. Jia, H.-X. Song, C.-P. Zhang, Org. Lett.,
2017, 19, 5454-5457.

Synthesis of Internal Alkynes through an Effective Tandem
Elimination-Hydrodebromination-Cross-Coupling of gem-Dibromoalkenes with
Halobenzenes
Y. Ji, N. Zhong, Z. Kang, G. Yan, M. Zhao,
Synlett, 2018, 29, 209-214.

One-Pot Conversion of Aldehydes and Aryl Halides to Disubstituted Alkynes
via Tandem Seyferth-Gilbert Homologation/Copper-Free Sonogashira Coupling
A. Sapegin, M. Krasavin, J. Org. Chem., 2019, 84,
8788-8795.

General, Robust, and Stereocomplementary Preparation of β-Ketoester Enol
Tosylates as Cross-Coupling Partners Utilizing TsCl-N-Methylimidazole Agents
H. Nakatsuji, K. Ueno, T. Misaki, Y. Tanabe, Org. Lett., 2008,
10, 2131-2134.

Single-Isomer Tetrasubstituted Olefins from Regioselective and
Stereospecific Palladium-Catalyzed Coupling of β-Chloro-α-iodo-α,β-unsaturated
Esters
A. B. Lemay, K. S. Vulic, W. W. Ogilvie, J. Org. Chem., 2006, 71, 3615-3618.

Palladium-Catalyzed Coupling of Terminal Alkynes with Benzyl Ammonium Salts
S. Xu, Z. Zhang, C. Han, W. Hu, T. Xiao, Y. Yuan, J. Zhao, J. Org. Chem., 2019, 84,
12157-12164.

Palladium-Catalyzed Carbonylative Sonogashira Coupling of Aryl Bromides
Using Near Stoichiometric Carbon Monoxide
K. T. Neumann, S. R. Laursen, A. T. Lindhardt, B. Bang-Andersen, T. Skrydstrup, Org. Lett., 2014,
16, 2216-2219.

Straightforward Novel One-Pot Enaminone and Pyrimidine Syntheses by
Coupling-Addition-Cyclocondensation Sequences
A. S. Karpov, T. J. J. Müller, Synthesis, 2003, 2815-2826.

Electrochemical Palladium-Catalyzed Oxidative Sonogashira Carbonylation of
Arylhydrazines and Alkynes to Ynones
Y. Wu, L. Zeng, H. Li, Y. Cao, J. Hu, M. Xu, R. Shi, H. Yi, A. Lei, J. Am. Chem. Soc.,
2021, 143, 12460-12466.

Ynamides are moisture-sensitive and prone to hydration especially under
acidic and heating conditions. An environmentally benign, robust Sonogashira
coupling
enables the synthesis of sulfonamide-based ynamides and arylynamines in water, using a readily available quaternary
ammonium salt as the surfactant.
L. Zhao, H. Yang, R. Li, Y. Tao, X.-F. Guo, E. A. Anderson, A. Whiting, N.
Wu, J. Org. Chem., 2021, 86,
1938-1947.

A domino intermolecular Sonogashira coupling of
2-(2-bromophenoxy)-1-phenylethan-1-ones/alkyl
2-(2-bromophenoxy)acetates/2-(2-bromophenoxy)acetonitrile/1-(2-bromophenoxy)propan-2-one
with terminal acetylenes followed by an intramolecular carbanion-yne cyclization
in a 5-exo-dig manner and subsequent double-bond isomerization
provide 2,3-disubstituted benzo[b]furans.
D. R. Kishore, G. Satyanarayana, J. Org. Chem., 2022, 87,
10158-10172.

Synthesis of Isoquinolines and Pyridines by the Palladium- and
Copper-Catalyzed Coupling and Cyclization of Terminal Acetylenes
K. R. Roesch, R. C. Larock,
Org. Lett., 1999, 1, 553-556.
