Further Information
Literature
Related Reactions
Hiyama Coupling
Kumada Coupling
Miyaura Borylation Reaction
Negishi Coupling
Stille Coupling
Suzuki Coupling

The scheme above shows the first published Suzuki Coupling, which is the palladium-catalysed cross coupling between organoboronic acid and halides. Recent catalyst and methods developments have broadened the possible applications enormously, so that the scope of the reaction partners is not restricted to aryls, but includes alkyls, alkenyls and alkynyls. Potassium trifluoroborates and organoboranes or boronate esters may be used in place of boronic acids. Some pseudohalides (for example triflates) may also be used as coupling partners.
Mechanism of the Suzuki Coupling
One difference between the Suzuki mechanism and that of the Stille Coupling is that the boronic acid must be activated, for example with base. This activation of the boron atom enhances the polarisation of the organic ligand, and facilitates transmetallation. If starting materials are substituted with base labile groups (for example esters), powdered KF effects this activation while leaving base labile groups unaffected.
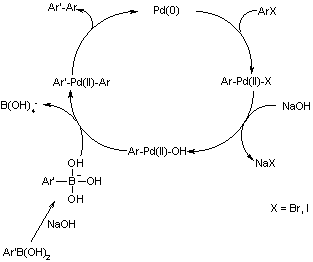
In part due to the stability, ease of preparation and low toxicity of the boronic acid compounds, there is currently widespread interest in applications of the Suzuki Coupling, with new developments and refinements being reported constantly.
Recent Literature

Alkyl-Alkyl Suzuki Cross-Couplings of Unactivated Secondary Alkyl Halides at
Room Temperature
B. Saito, G. C. Fu, J. Am. Chem. Soc., 2007,
129, 9602-9603.

Boronic Acids: New Coupling Partners in Room-Temperature Suzuki Reactions of
Alkyl Bromides. Crystallographic Characterization of an Oxidative-Addition
Adduct Generated under Remarkably Mild Conditions
J. H. Kirchhoff, M. R. Netherton, I. D. Hill, G. C. Fu, J. Am. Chem. Soc.,
2002,
124, 13662-13663.

B-Alkyl sp3-sp2 Suzuki-Miyaura Couplings under Mild Aqueous Micellar
Conditions
N. R. Lee, R. T. H. Linstadt, D. J. Gloisten, F. Gallou, B. H. Lipshutz, Org. Lett.,
2018, 20, 2902-2905.

Rational Exploration of N-Heterocyclic Carbene (NHC) Palladacycle Diversity:
A Highly Active and Versatile Precatalyst for Suzuki-Miyaura Coupling Reactions
of Deactivated Aryl and Alkyl Substrates
G.-R. Peh, E. A. B. Kantchev, J.-C. Er, J. Y. Ying, Chem. Eur. J., 2010,
14, 4010-4017.

Stereoconvergent Amine-Directed Alkyl-Alkyl Suzuki Reactions of Unactivated
Secondary Alkyl Chlorides
Z. Lu, A. Wilsily, G. C. Fu, J. Am. Chem. Soc., 2011,
133, 8154-8157.

A Catalytic Enantiotopic-Group-Selective Suzuki Reaction for the
Construction of Chiral Organoboronates
C. Sun, B. Potter, J. P. Morken, J. Am. Chem. Soc., 2014,
136, 6534-6537.

Tetraphosphine/palladium catalysed Suzuki cross-coupling reactions of aryl
halides with alkylboronic acids
I. Kondolff, H. Doucet, M. Santelli, Tetrahedron, 2004, 60,
3813-3818.

Suzuki-Miyaura Cross-Coupling Reactions of Primary Alkyltrifluoroborates
with Aryl Chlorides
S. D. Dreher, S.-E. Lim, D. L. Sandrock, G. A. Molander, J. Org. Chem., 2009,
74, 3626-3631.

A Concise and Atom-Economical Suzuki-Miyaura Coupling Reaction Using
Unactivated Trialkyl- and Triarylboranes with Aryl Halides
H. Li, Y.-L. Zhong, C.-y. Chen, A. E. Ferraro, D. Wang, Org. Lett.,
2015,
17, 3616-3619.

[(NHC)PdCl2(Aniline)] Complexes: Easily Synthesized, Highly Active
Pd(II)-NHC Precatalysts for Cross-Coupling Reactions
Q. Xia, S. Shi, P. Gao, R. Lalancette, R. Szostak, M. Szostak, J. Org. Chem., 2021, 86,
15650-15657.

Stereospecific Pd-Catalyzed Cross-Coupling Reactions of Secondary Alkylboron
Nucleophiles and Aryl Chlorides
L. Li, S. Zhao, A. Joshi-Pangu, M. Diane, M. R. Biscoe,
J. Am. Chem. Soc., 2014,
136, 14027-14030.

Pd(N,N-Dimethyl β-alaninate)2 as a High-Turnover-Number, Phosphine-Free
Catalyst for the Suzuki Reaction
X. Cui, T. Qin, J.-R. Wang, L. Liu, Q.-X. Guo, Synthesis, 2007,
393-399.

Guanidine/Pd(OAc)2-Catalyzed Room Temperature Suzuki
Cross-Coupling Reaction in Aqueous Media under Aerobic Conditions
S. Li, Y. Lin, J. Cao, S. Zhang, J. Org. Chem., 2007,
72, 4067-4072.

Aqueous Suzuki-Miyaura Coupling with Ultralow Palladium Loading and Simple
Product Separation
W. Yang, W.-D. Xu, J.-J. Ning, Z.-G. Ren, D. J. Young, Synlett, 2020,
31,
1303-1307.

Pd2(dba)3/P(t-Bu)3 catalyzes Suzuki
cross-coupling of arylboronic acids with a wide range of aryl and vinyl halides,
including chlorides, typically at room temperature, while the combination of
Pd(OAc)2/PCy3 is suitable for a diverse array of aryl and
vinyl triflates. Together, these two catalyst systems cover a broad spectrum of
commonly encountered substrates for Suzuki couplings.
A. F. Littke, C. Dai, G. C. Fu, J. Am. Chem. Soc., 2000,
122, 4020-4028.

Liquid-Assisted Grinding Accelerating: Suzuki-Miyaura Reaction of Aryl
Chlorides under High-Speed Ball-Milling Conditions
Z.-J. Jiang, Z.-H. Li, J.-B. Yu, W.-K. Su, J. Org. Chem.,
2016, 81, 10049-10055.

[Bmim]PF6-Promoted Ligandless Suzuki-Miyaura Coupling Reaction of Potassium
Aryltrifluoroborates in Water
L. Liu, Y. Dong, B. Pang, J. Ma, J. Org. Chem., 2014,
79, 7193-7198.

Sterically Demanding, Water-Soluble Alkylphosphines as Ligands for High
Activity Suzuki Coupling of Aryl Bromides in Aqueous Solvents
K. H. Shaughnessy, R. S. Booth, Org. Lett., 2001,
3, 2757-2759.

Convenient and Efficient Suzuki-Miyaura Cross-Coupling Catalyzed by a
Palladium/Diazabutadiene System
G. A. Grasa, A. C. Hillier, S. P. Nolan,
Org. Lett., 2001, 3, 1077-1080.

An Indefinitely Air-Stable σ-NiII Precatalyst for Quantitative
Cross-Coupling of Unreactive Aryl Halides and Mesylates with Aryl
Neopentylglycolboronates
J. Malineni, R. L. Jezorek, N. Zhang, V. Percec, Synthesis, 2016,
48, 2795-2807.

Copper-Catalyzed Suzuki-Miyaura Coupling of Arylboronate Esters:
Transmetalation with (PN)CuF and Identification of Intermediates
S. K. Gurung, S. Thapa, A. Kafle, D. A. Dickie, R. Giri, Org. Lett., 2014,
16, 1264-1267.

The Rational Design and Synthesis of Water-Soluble Thiourea Ligands for
Recoverable Pd-Catalyzed Aerobic Aqueous Suzuki-Miyaura Reactions at Room
Temperature
W. Chen, X.-Y. Lu, B.-Hua, W.-g. Yu, Z.-n. Zhou, Y. Hu, Synthesis, 2018, 50,
1499-1510.

A Cyclobutene-1,2-Bis(imidazolium) Salt as Efficient Precursor of
Palladium-Catalyzed Room-Temperature Suzuki-Miyaura Reactions
A. Rahimi, A. Schmidt, Synlett, 2010,
1327-1330.

An Extremely Active and General Catalyst for Suzuki Coupling Reaction of
Unreactive Aryl Chlorides
D.-H. Lee, M.-J. Jin, Org. Lett., 2011,
13, 252-255.

A Triarylphosphine Ligand Bearing Dodeca(ethylene glycol) Chains: Enhanced
Efficiency in the Palladium-Catalyzed Suzuki-Miyaura Coupling Reaction
T. Fujihara, S. Yoshida, J. Terao, Y. Tsuji, Org. Lett., 2009,
11, 2121-2124.

A New Family of Tunable Indolylphosphine Ligands by One-Pot Assembly and
Their Applications in Suzuki-Miyaura Coupling of Aryl Chlorides
C. M. So, C. C. Yeung, C. P. Lau, F. Y. Kwong, J. Org. Chem., 2008,
73, 7803-7806.

An Active, General, and Long-Lived Palladium Catalyst for Cross-Couplings of
Deactivated (Hetero)aryl Chlorides and Bromides with Arylboronic Acids
T. Hoshi, T. Honma, A. Mori, M. Konishi, T. Sato, H. Hagiwara, T. Suzuki, J. Org. Chem., 2013,
78, 11513-11524.

Biphenylene-Substituted Ruthenocenylphosphine for Suzuki-Miyaura Coupling of
Aryl Chlorides
T. Hoshi, T. Nakazawa, I. Saitoh, Y. Mori, T. Suzuki, J.-i. Sakai, H.
Hagiwara, S. Akai, Org. Lett., 2008,
10, 2063-2066.

Scope of the Two-Step, One-Pot Palladium-Catalyzed Borylation/Suzuki
Cross-Coupling Reaction Utilizing Bis-Boronic Acid
G. A. Molander, S. L. J. Trice, S. M. Kennedy, J. Org. Chem., 2012,
77, 8678-8688.

Facile Synthesis of Highly Stable Gold Nanoparticles and Their Unexpected
Excellent Catalytic Activity for Suzuki-Miyaura Cross-Coupling Reaction in Water
J. Han, Y. Liu, R. Guo, J. Am. Chem. Soc., 2009,
131, 2060-2061.

Biscarboxy-Functionalized Imidazole and Palladium as Highly Active Catalytic
System in Protic Solvents: Methanol and Water
R. Martínez, I. M. Pastor, M. Yus, Synthesis, 2014, 46,
2965-2975.

Room-Temperature Suzuki-Miyaura Couplings in Water Facilitated by Nonionic
Amphiphiles
B. H. Lipshutz, T. B. Petersen, A. R. Abela, Org. Lett., 2008,
10, 1333-1336.

Robust Acenaphthoimidazolylidene Palladium Complexes: Highly Efficient
Catalysts for Suzuki-Miyaura Couplings with Sterically Hindered Substrates
T. Tu, Z. Sun, W. Fang, M. Xu, Y. Zhou, Org. Lett., 2012,
14, 4250-4253.

Solvent-Free Microwave-Assisted Suzuki-Miyaura Coupling Catalyzed by
PEPPSI-iPr
P. Nun, J. Martinez, F. Lamaty, Synlett, 2009,
1761-1764.

Cross-Coupling and Dehalogenation Reactions Catalyzed by (N-Heterocyclic
carbene)Pd(allyl)Cl Complexes
O. Navarro, H. Kaur, P. Mahjoor, S. P. Nolan, J. Org. Chem., 2004,
69, 3173-3180.

An N-Heterocyclic Carbene Ligand with Flexible Steric Bulk Allows Suzuki
Cross-Coupling of Sterically Hindered Aryl Chlorides at Room Temperature
G. Altenhoff, R. Goddard, C. W. Lehmann, F. Glorius, Angew. Chem. Int. Ed.,
2003, 42, 3690-3693.

Ferrocenyl Monophosphine Ligands: Synthesis and Applications in the
Suzuki-Miyaura Coupling of Aryl Chlorides
C. Baillie, L. Zhang, J. Xiao, J. Org. Chem., 2004,
69, 7779-7782.

Reusable and Efficient Pd(OAc)2/TBAB/PEG-400 System for Suzuki-Miyaura
Cross-Coupling Reaction under Ligand-Free Conditions
W.-J. Liu, Y.-X. Xie, Y. Liang, J.-H. Li,
Synthesis, 2006, 860-864.

Phosphine-Free Palladium Acetate Catalyzed Suzuki Reaction in Water
L. Liu, Y. Zhang, Y. Wang, J. Org. Chem., 2005,
70, 6122-6125.

Cyrene as a Bio-Based Solvent for the Suzuki-Miyaura Cross-Coupling
K. L. Wilson, J. Murray, C. Jamieson, A. J. B. Watson,
Synlett, 2018, 29, 650-654.

Synthesis and Characterization of R2PN=P(iBuNCH2CH2)3N: A New Bulky
Electron-Rich Phosphine for Efficient Pd-Assisted Suzuki-Miyaura Cross-Coupling
Reactions
J. V. Kingston, J. G. Verkade, J. Org. Chem., 2007,
72, 2816-2822.

Modified (NHC)Pd(allyl)Cl (NHC = N-Heterocyclic Carbene) Complexes
for Room-Temperature Suzuki-Miyaura and Buchwald-Hartwig Reactions
N. Marion, O. Navarro, J. Mei, E. D. Stevens, N. M. Scott, S. P. Nolan, J. Am. Chem. Soc., 2006, 128, 4101-4111.

Aryl Boronic Esters Are Stable on Silica Gel and Reactive under
Suzuki-Miyaura Coupling Conditions
N. Oka, T. Yamada, H. Sajiki, S. Akai, T. Ikawa, Org. Lett.,
2022, 24, 3510-3514.

A Highly Active Catalyst for Suzuki-Miyaura Cross-Coupling Reactions of
Heteroaryl Compounds
K. L. Billingsley, K. W. Anderson, S. L. Buchwald, Angew. Chem. Int. Ed., 2006, 45, 3484-3488.

Palladium catalyzed Suzuki-Miyaura coupling with aryl chlorides using a
bulky phenanthryl N-heterocyclic carbene ligand
C. Song, Y. Ma, Q. Chai, C. Ma, W. Jiang, M. B. Andrus, Tetrahedron, 2005,
61, 7438-7446.

PVC-Supported Palladium Nanoparticles: An Efficient Catalyst for Suzuki
Cross-Coupling Reactions at Room Temperature
M. Samarasimhareddy, G. Prabhu, T. M. Vishwanatha, V. V. Sureshbabu, Synthesis, 2013, 45,
1201-1206.

Recyclable Catalysts for Suzuki-Miyaura Cross-Coupling Reactions at Ambient
Temperature Based on a Simple Merrifield Resin Supported
Phenanthroline-Palladium(II) Complex
J. Yang, P. Li, L. Wang, Synthesis, 2011,
1295-1301.

Immobilization of Dipyridyl Complex to Magnetic Nanoparticle via Click
Chemistry as a Recyclable Catalyst for Suzuki Cross-Coupling Reactions
G. Lv, W. Mai, R. Jin, L. Gao, Synlett, 2008,
1418-1422.

PdEDTA Held in an Ionic Liquid Brush as a Highly Efficient and Reusable
Catalyst for Suzuki Reactions in Water
J.-F. Wei, J. Jiao, J.-J. Feng, J. Lv, X.-R. Zhang, X.-Y. Shi, Z.-G. Chen, J. Org. Chem., 2009,
74, 5967-5974.

Palladium-Imidazolium Carbene Catalyzed Aryl, Vinyl, and Alkyl
Suzuki-Miyaura Cross Coupling
M. B. Andrus, C. Song, Org. Lett., 2001,
3, 3761-3764.

Overcoming the Naphthyl Requirement in Stereospecific Cross-Couplings to
Form Quaternary Stereocenters
J. Xu, O. P. Bercher, M. P. Watson, J. Am. Chem. Soc.,
2021, 143, 8608-8613.

Vinyl Thianthrenium Tetrafluoroborate: A Practical and Versatile Vinylating
Reagent Made from Ethylene
F. Juliá, J. Yan, F. Paulus, T. Ritter, J. Am. Chem. Soc.,
2021, 143, 12992-12998.

Ligand Effects on the Stereochemical Outcome of Suzuki-Miyaura Couplings
G.-P. Lu, K. R. Voigtritter, C. Cai, B. H. Lipshutz, J. Org. Chem., 2012,
77, 3700-3703.

Synthesis of E-Alkyl Alkenes from Terminal Alkynes via Ni-Catalyzed
Cross-Coupling of Alkyl Halides with B-Alkenyl-9-borabicyclo[3.3.1]nonanes
T. Di Franco, A. Epenoy, X. Hu, Org. Lett.,
2015,
17, 4910-4913.

Highly E-Selective, Stereoconvergent Nickel-Catalyzed Suzuki-Miyaura
Cross-Coupling of Alkenyl Ethers
G.-M. Ho, H. Sommer, I. Marek,
Org. Lett., 2019, 21, 2913-2917.

MIDA-Vinylsilanes: Selective Cross-Couplings and Applications to the
Synthesis of Functionalized Stilbenes
M. G. McLaughlin, C. A. McAdam, M. J. Cook, Org. Lett.,
2015,
17, 10-13.

An Efficient and Recyclable Magnetic-Nanoparticle-Supported Palladium
Catalyst for the Suzuki Coupling Reactions of Organoboronic Acids with Alkynyl
Bromides
X. Zhang, P. Li, Y. Ji, L. Zhang, L. Wang, Synthesis, 2011,
2975-2983.

Efficient Palladium-Catalyzed Cross-Coupling Reaction of Alkynyl Halides
with Organoboronic Acids under Aerobic Conditions
J.-S. Tang, M. Tian, W.-B. Sheng, C.-C. Guo, Synthesis, 2012, 44,
541-546.

Suzuki-Miyaura Coupling of Alkynylboronic Esters Generated in Situ from
Acetylenic Derivatives
A.-S. Castanet, F. Colobert, T. Schlama,
Org. Lett., 2000, 2, 3559-3561.

Highly Active Catalyst for the Heterogeneous Suzuki-Miyaura Reaction:
Assembled Complex of Palladium and Non-Cross-Linked Amphiphilic Polymer
Y. M. A. Yamada, K. Takeda, H. Takashashi, S. Ikegami, J. Org. Chem.,
2003,
68, 7733-7741.

New Catalysts for Suzuki-Miyaura Coupling Reactions of
Heteroatom-Substituted Heteroaryl Chlorides
A. S. Guram, X. Wang, E. E. Bunel, M. M. Faul, R. D. Larsen, M. J. Martinelli, J. Org. Chem., 2007,
72, 5104-5112.

C2-Symmetric Bis-Hydrazones as Ligands in the Asymmetric Suzuki−Miyaura
Cross-Coupling
A. Bermejo, A. Ros, R. Fernández, J. M. Lassaletta, J. Am. Chem. Soc., 2008,
130, 15798-15799.

General Reaction Conditions for the Palladium-Catalyzed Vinylation of Aryl
Chlorides with Potassium Alkenyltrifluoroborates
E. Alacid, C. Nájera, J. Org. Chem., 2009,
74, 8191-8195.

Stereoselective Enol Tosylation: Preparation of Trisubstituted
α,β-Unsaturated Esters
J. Baxter, D. Steinhuebel, M. Palucki, I. W. Davies,
Org. Lett., 2005, 7, 215-218.

N-Vinylpyridinium and -ammonium Tetrafluoroborate Salts: New Electrophilic
Coupling Partners for Pd(0)-Catalyzed Suzuki Cross-Coupling Reactions
K. R. Buszek, N. Brown, Org. Lett., 2007,
9, 707-710.

Suzuki-Miyaura Cross-Coupling of Potassium Trifluoroboratohomoenolates
G. A. Molander, D. E. Petrillo, Org. Lett., 2008,
10, 1795-1798.

Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling of Oxygen-Substituted
Allylboronates with Aryl/Vinyl (Pseudo)Halides
J. Li, X. Zhang, Y. Yao, Y. Gao, W. Yang, W. Zhao, J. Org. Chem., 2022, 87,
6951-6959.

Palladium-Catalyzed One-Pot Cross-Coupling of Phenols Using
Nonafluorobutanesulfonyl Fluoride
T. Ikawa, K. Saito, S. Akai, Synlett, 2012, 23,
2241-2246.

A family of indolyl phosphine ligands was applied to Suzuki-Miyaura
cross-coupling of aryl tosylates with boronic acids, trifluoroborate salts, and
boronate esters. Catalyst loading can be reduced to 0.2 mol % for the coupling
of nonactivated aryl tosylates.
C. M. So, C. P. Lau, A. S. C. Chan, F. Y. Kwong, J. Org. Chem., 2008,
73, 7731-7734.

A General Palladium Catalyst System for Suzuki-Miyaura Coupling of Potassium
Aryltrifluoroborates and Aryl Mesylates
W. K. Chow, C. M. So, C. P. Lau, F. Y. Kwong, J. Org. Chem., 2010,
75, 5109-5112.

Ni(COD)2/PCy3 Catalyzed Cross-Coupling of Aryl and Heteroaryl
Neopentylglycolboronates with Aryl and Heteroaryl Mesylates and Sulfamates in
THF at Room Temperature
P. Leowanawat, N. Zhang, A.-M. Remerita, B. M. Rosen, V. Percec, J. Org. Chem., 2011,
76, 9946-9955.

The First General Palladium Catalyst for the Suzuki-Miyaura and Carbonyl
Enolate Coupling of Aryl Arenesulfonates
H. N. Nguyen, X. Huang, S. L. Buchwald, J. Am. Chem. Soc., 2003, 125, 11818-11819.

Synthesis of Biaryl Derivatives via a Magnetic Pd-NPs-Catalyzed One-Pot
Diazotization-Cross-Coupling Reaction
Y. Zong, J. Hu, P. Sun, X. Jiang, Synlett, 2012, 23,
2393-2396.

The Suzuki-Miyaura Coupling of Nitroarenes
M. R. Yadav, M. Nagaoka, M. Kashihara, R.-L. Zhong, T. Miyazaki, S. Sakaki, Y.
Nakao, J. Am. Chem. Soc., 2017,
139, 9423-9426.

Palladium-Catalyzed Cross-Coupling Reactions of Potassium
Alkenyltrifluoroborates with Organic Halides in Aqueous Media
E. Alacid, C. Nájera, J. Org. Chem., 2009,
74, 2321-2327.

The use of bisphosphine ligands with a large P-Pd-P bite angle allowed to
synthesize Z-chlorinated internal alkenes in good yields by a selective
Suzuki-Miyaura monocoupling process of 9-alkyl-9-BBN with
1,1-dichloro-1-alkenes. These monochlorinated olefins could be further
transformed providing stereospecifically trisubstituted olefins.
F. Liron, C. Fosse, A. Pernolet, E. Roulland, J. Org. Chem., 2007,
72, 2220-2223.

Stereoselective Cross-Coupling Reaction of 1,1-Diboryl-1-alkenes with
Electrophiles: A Highly Stereocontrolled Approach to 1,1,2-Triaryl-1-alkenes
M. Shimizu, C. Nakamaki, K. Shimono, M. Schelper, T. Kurahashi, T. Hiyama, J. Am. Chem. Soc., 2005, 127, 12506-12507.

Suzuki-Miyaura Cross-Coupling Reactions of Potassium Alkenyltrifluoroborates
G. A. Molander, C. R. Bernardi, J. Org. Chem.,
2002, 67, 8424-8429.

Mechanochemical Synthesis of Ketones via Chemoselective Suzuki-Miyaura
Cross-Coupling of Acyl Chlorides
J. Zhang, P. Zhang, Y. Ma, M. Szostak, Org. Lett.,
2022, 24, 2338-2343.

Evaluation of Cyclic Amides as Activating Groups in N-C Bond Cross-Coupling:
Discovery of N-Acyl-δ-valerolactams as Effective Twisted Amide Precursors for
Cross-Coupling Reactions
Md. M. Rahman, D. J. Pyle, E. Bisz, B. Dziuk, K. Ejsmont, R. Lalancette, Q.
Wang, H. Chen, R. Szostak, M. Szostak, J. Org. Chem., 2021, 86,
10455-10466.

General Method for the Suzuki-Miyaura Cross-Coupling of Primary
Amide-Derived Electrophiles Enabled by [Pd(NHC)(cin)Cl] at Room Temperature
P. Lei, G. Meng, Y. Ling, J. An, S. P. Nolan, M. Szostak, Org. Lett.,
2017, 19, 6510-6513.

Pd-PEPPSI: Pd-NHC Precatalyst for Suzuki-Miyaura Cross-Coupling Reactions of
Amides
P. Lei, G. Meng, Y. Ling, J. An, M. Szostak, J. Org. Chem.,
2017, 82, 6638-6646.

Triflamides: Highly Reactive, Electronically Activated N-Sulfonyl
Amides in Catalytic N-C(O) Amide Cross-Coupling
S. Shi, R. Lalancette, R. Szostak, M. Szostak, Org. Lett., 2019, 21,
1253-1257.

Sterically Hindered Ketones via Palladium-Catalyzed Suzuki-Miyaura
Cross-Coupling of Amides by N-C(O) Activation
C. Liu, R. Lalancette, R. Szostak, M. Szostak,
Org. Lett., 2019, 21, 7976-7981.

Palladium-Catalyzed Suzuki-Miyaura Coupling of Aryl Esters
T. B. Halima, W. Zhang, I. Yalaoui, X. Hong, Y.-F. Yang, K. N. Houk, S. G.
Newman, J. Am. Chem. Soc., 2017,
139, 1311-1318.

Palladacycle-Catalyzed Carbonylative Suzuki-Miyaura Coupling with High
Turnover Number and Turnover Frequency
P. Gautam, B. M. Bhanage, J. Org. Chem.,
2015,
80, 7810-7815.

Carbonylative Suzuki-Miyaura Coupling Reactions of Aryl Iodides with Readily
Available Polymer-Immobilized Palladium Nanoparticles
T. Yasukawa, Z. Zhu, Y. Yamashita, S. Kobayashi, Synlett, 2021,
32,
502-504.

Stereospecific Acylative Suzuki-Miyaura Cross-Coupling: General Access to
Optically Active α-Aryl Carbonyl Compounds
B. Roh, A. O. Farh, M. Kim, T. Feoktistova, F. Moeller, K. D. Kim, P. H.-Y.
Cheong, H. G. Lee, J. Am. Chem. Soc.,
2023, 145, 7075-7083.

Deaminative Arylation of Amino Acid-derived Pyridinium Salts
M. E. Hoerrner, K. M. Baker, C. H. Basch, E. M. Bampo, M. P. Watson,
Org. Lett., 2019, 21, 7356-7360.

Switchable Selectivity in the Pd-Catalyzed Alkylative Cross-Coupling of
Esters
J. Masson-Makdissi, J. K. Vandavasi, S. G. Newman, Org. Lett.,
2018, 20, 4094-4098.

Pd/NHC-Catalyzed Enantiospecific and Regioselective Suzuki-Miyaura Arylation
of 2-Arylaziridines: Synthesis of Enantioenriched 2-Arylphenethylamine
Derivatives
Y. Takeda, Y. Ikeda, A. Kuroda, S. Tanaka, S. Minataka, J. Am. Chem. Soc., 2014,
136, 8544-8547.

Desymmetrization of Vicinal Bis(boronic) Esters by Enantioselective
Suzuki-Miyaura Cross-Coupling Reaction
M. Zhang, P. S. Lee, C. Allais, R. A. Singer, J. P. Morken, J. Am. Chem. Soc.,
2023, 145, 8308-8313.
