Further Information
Literature
Related Reactions
Grignard
Reaction
Reformatsky Reaction
Weinreb Ketone Synthesis
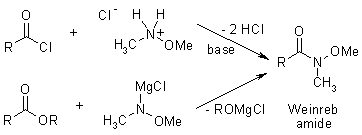
The reaction of esters and carboxylic acid chlorides with organolithium and organomagnesium compounds does not lead to ketones in high yields, because the intermediate ketones are still highly reactive toward the organometallic reagent. However, after derivatisation to the corresponding Weinreb Amide, reaction with organometallics does give the desired ketones, as the initial adduct is stabilized and doesn't undergo further reaction.

Mechanism of the Weinreb Ketone Synthesis
With the usual reaction of organometallic reagents with acid derivatives (ester or acid chloride), the starting materials can add two equivalents of organometallic compound. The ketone generated after the first addition is quite reactive, and there is quite no selectivity between it and the starting acid derivative:


The organometallic adducts of Weinreb Amides are able to form stable chelates, and do not regenerate an electrophilic carbonyl group in situ for further reaction:

Aqueous work up liberates the ketone from this chelate:

Recent Literature

Benzylic Aroylation of Toluenes Mediated by a LiN(SiMe3)2/Cs+
System
Y. Gu, Z. Zhang, Y.-E. Wang, Z. Dai, Y. Yuan, D. Xiong, J. Li, P. J. Walsh,
J. Mao, J. Org. Chem., 2022, 87,
406-418.

One-Step Synthesis of 1-Chloro-3-arylacetone Derivatives from Arylacetic
Acids
M. J. Zacuto, R. F. Dunn, M. Figus, J. Org. Chem., 2014,
79, 8917-8925.

Modified Shapiro Reactions with Bismesitylmagnesium As an Efficient Base
Reagent
W. J. Kerr, A. J. Morrison, M. Pazicky, T. Weber, Org. Lett., 2012,
14, 2250-2253.

Novel Sequential Process from N-Methoxyamides and Vinyl Grignard
Reagents: New Synthesis of β-Aminoketones
A. Gomtsyan, R. J. Koenig, C.-H. Lee, J. Org. Chem., 2001,
66, 3613-3616.

An Easy and Convenient Synthesis of Weinreb Amides and Hydroxamates
L. De Luca, G. Giacomelli, M. Taddei, J. Org. Chem., 2001, 66, 2534-2537.

Synthesis of α,β-Unsaturated α'-Haloketones through the Chemoselective
Addition of Halomethyllithiums to Weinreb Amides
V. Pace, L. Castoldi, W. Holzer, J. Org. Chem., 2013,
78, 7764-7770.

Accessing Diverse Cross-Benzoin and α-Siloxy Ketone Products via Acyl
Substitution Chemistry
C. N. Larcombe, L. R. Malins, J. Org. Chem., 2022, 87,
9408-9413.
