Categories: C=O Bond Formation > Synthesis of aldehydes >
Synthesis of aldehydes by cleavage of alkenes
| Related |
|
|
Name Reactions
Recent Literature
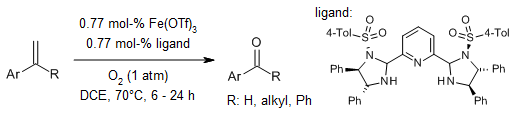
A mild and operationally simple protocol for the selective aerobic oxidation of
aromatic olefins to carbonyl compounds is catalyzed by a Fe(III) species bearing
a pyridine bisimidazoline ligand at 1 atm of O2. The method cleaves
α- and β-substituted styrenes to afford benzaldehydes and aromatic ketones in
high yields with excellent chemoselectivity and very good functional group
tolerance.
A. Gonzalez-de-Castro, J. Xiao, J. Am. Chem. Soc., 2015,
137, 8206-8218.

For a selective oxidation of olefins, in particular, aromatic olefins to
carbonyls, stoichiometric toxic oxidants or a high-cost catalyst is required. A
practical light-enabled oxidation of olefins using H2O2 as a clean and low-toxic oxidant
provides a broad scope of carbonyls in high yield without catalyst.
W. Yu, Z. Zhao,
Org. Lett., 2019, 21, 7713-7716.

Sodium benzene sulfinate catalyzed a visible-light-driven aerobic oxidative
cleavage of olefins to provide the corresponding aldehydes and ketones under
transition-metal-free conditions. Notably, α-halo-substituted styrenes proceeded
with photoinduced oxidation to finally afford α-halo-acetophenones with halogen
migration.
Y.-X. Chen, J.-T. He, M.-C. Wu, Z.-L. Liu, K. Tang, P.-J. Xia, K. Chen, H.-Y.
Xiang, X.-Q. Chen, H. Yang, Org. Lett.,
2022, 24, 3920-3925.

A ruthenium catalyst bearing a fused π-conjugated imidazo[1,2-a][1,8]naphthyridine-based
abnormal N-heterocyclic carbene ligand enables a selective oxidation of C═C
bonds in a broad range of substrate to aldehydes and C≡C bonds to α-diketones in
an EtOAc/CH3CN/H2O solvent mixture at room temperature.
P. Daw, R. Petakamsetty, A. Sarbajna, S. Laha, R. Ramapanicker, J. K. Bera,
J. Am. Chem. Soc., 2014,
136, 13987-13990.

Osmium tetroxide has been microencapsulated in a polyurea matrix. These
microcapsules have been effectively used as recyclable catalysts in the
dihydroxylation and the oxidative cleavage of olefins.
S. V. Ley, C. Ramarao, A.-L. Lee, N. Ostergaard, S. C. Smith, I. M. Shirley,
Org. Lett., 2003, 5, 185-187.

The use of PhI(OAc)2 in dichloromethane enables a clean oxidative
cleavage of 1,2-diols to aldehydes. In the presence of OsO4 as
catalyst, NMO and 2,6-lutidine, olefinic bonds can be cleaved in acetone/water
to yield the corresponding carbonyl compounds.
K. C. Nicolaou, V. A. Adsool, C. R. H. Hale, Org. Lett., 2010,
12, 1552-1555.

A gold(I)-catalyzed oxidative cleavage of alkenes with tert-butyl
hydrogenperoxide (TBHP) as the oxidant in the presence of neocuproine
afforded ketones or aldehydes as products.
D. Xing, B. Guan, G. Cai, Z. Fang, L. Yang, Z. Shi, Org. Lett.,
2006, 8, 693-696.

A series of symmetrical and unsymmetrical stilbenes bearing electron-withdrawing
groups were oxidatively cleaved to the corresponding aldehydes in high yield by
electrocatalytic anodic oxidation employing a high oxidation potential
triphenylamine electrocatalyst. The oxidations apparently involve the
corresponding 1,2-diols, which are also converted to aldehydes in high yield
under the same conditions.
X. Wu, A. P. Davis, A. J. Fry, Org. Lett., 2007,
9, 5633-5636.

A catalytic amount of a composite material, RuO2/BaTi4O9,
in combination with NaIO4 in EtOAc-H2O has been shown to
efficiently cleave alkenes, affording ketones, aldehydes and/or carboxylic acids
in high yields.
H. Okumoto, K. Ohtsuko, S. Banjoya, Synlett, 2007,
3201-3205.

Specific oxidation protocols have been developed for the cleavage of styrenes,
aliphatic olefins, and terminal aliphatic olefins to carbonyl compounds with
ruthenium trichloride as catalyst. Olefins that are not fully substituted are
converted to aldehydes rather than carboxylic acids.
D. Yang, C. Zhang, J. Org. Chem., 2001, 66, 4814-4818.

The oxidative cleavage of C=C bonds adjacent to aryl and alkyl moieties was
efficiently achieved with monoacetylated bishydroperoxides. Base-mediated
fragmentation of monoacetylated bishydroperoxides generates singlet molecular
oxygen as active oxidant in situ. All the reactions furnished the respective
carbonyl compounds in good yields at room temperature within short reaction
times.
D. Azarifar, Z. Najminejad, Synlett, 2013, 24,
1377-1382.

A simple, efficient, and environmentally beneficient disulfide-catalyzed
photocatalytic regioselective oxidative cleavage of 1-arylbutadienes to
cinnamaldehydes offers mild reaction conditions, excellent regioselectivity, and
compatibility with a wide range of functional groups.
R. A. Fernandes, P. Kumar, A. Bhowmik, D. A. Gorve, Org. Lett.,
2022, 24, 3436-3439.

Iron(III) sulfate mediates a simple and efficient regioselective oxidative
cleavage of 1-arylbutadienes in the presence of oxygen. The reaction offers good
yields, excellent regioselectivity, and good functional group tolerance.
A. Bhowmik, R. A. Fernandes,
Org. Lett., 2019, 21, 9203-9207.