Categories: C=O Bond Formation > Synthesis of aldehydes >
Synthesis of aldehydes
Name Reactions
Recent Literature

The use of tert-butyl nitrite as the co-catalyst in a 2-azaadamantane-N-oxyl
(AZADO)- and 9-azanoradamantane-N-oxyl (nor-AZADO)-catalyzed efficient
aerobic oxidation of primary alcohols in MeCN instead of the previously reported
AcOH provides the corresponding aldehydes selectively. The addition of saturated
aqueous NaHCO3 after the completion of the reaction suppresses the
overoxidation of the product during the workup.
M. Shibuya, K. Furukawa, Y. Yamamoto,
Synlett, 2017, 28, 1554-1557.

Cu/TEMPO catalyst systems show reduced reactivity in aerobic oxidation of
aliphatic and secondary alcohols. A catalyst system consisting of (MeObpy)CuOTf
and ABNO mediates aerobic oxidation of primary, secondary allylic, benzylic, and
aliphatic alcohols with nearly equal efficiency. The catalyst exhibits broad
functional group compatibility, and most reactions are complete within 1 h at
room temperature using ambient air as oxidant.
J. E. Steves, S. S. Stahl, J. Am. Chem. Soc., 2013,
135, 15742-15745.

A (bpy)CuI/TEMPO catalyst system enables an efficient and selective aerobic
oxidation of a broad range of primary alcohols, including allylic, benzylic, and
aliphatic derivatives, to the corresponding aldehydes using readily available
reagents, at room temperature with ambient air as the oxidant. The catalyst
system is compatible with a wide range of functional groups and shows a high
selectivity for 1° alcohols.
J. M. Hoover, S. S. Stahl, J. Am. Chem. Soc., 2011,
133, 16901-16910.

The combination of Fe(NO3)3·9H2O and
9-azabicyclo[3.3.1]nonan-N-oxyl enables an efficient aerobic oxidation of
a broad range of primary and secondary alcohols to the corresponding aldehydes
and ketones at room temperature with ambient air as the oxidant.
L. Wang, S. Shang, G. Li, L. Ren, Y. Lv, S. Gao, J. Org. Chem.,
2016,
81, 2189-2193.

An efficient bismuth tribromide catalyzed oxidation of various alcohols with
aqueous hydrogen peroxide provides carbonyl compounds in good yields.
M.-k. Han, S. Kim, S. T. Kim, J. C. Lee,
Synlett, 2015, 26, 2434-2436.

Oxidation of primary and secondary alcohols, using catalytic amounts of TEMPO
and tetra-n-butylammonium bromide in combination with periodic acid and
wet alumina in dichloromethane is compatible with a broad range of functional
groups and acid-sensitive protecting groups. The system also enables a
chemoselective oxidation of secondary alcohols in the presence of primary
alcohols.
M. Attoui, J.-M. Vatèle,
Synlett, 2014, 25, 2923-2927.
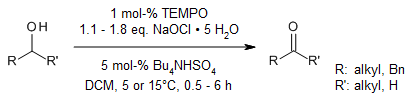
Sodium hypochlorite pentahydrate crystals with very low NaOH and NaCl contents
oxidize primary and secondary alcohols to the corresponding aldehydes and
ketones in the presence of TEMPO/Bu4NHSO4 without pH
adjustment. This new oxidation method is also applicable to sterically hindered
secondary alcohols.
T. Okada, T. Asawa, Y. Sugiyama, M. Kirihara, T. Iwai, Y. Kimura, Synlett, 2014, 25,
596-598.

The choline- and peroxydisulfate-based environmentally benign biodegradable
oxidizing task-specific ionic liquid (TSIL) choline peroxydisulfate (ChPS) was
synthesized and characterized. This reagent enables a selective oxidation of
alcohols to aldehydes/ketones in very good yields and short reaction time under
solvent-free mild reaction conditions without overoxidation to acid.
B. L. Gadilohar, H. S. Kumbhar, G. S. Shankarling, Ind. Eng. Chem. Res., 2014,
53, 19010-19018.

Silica-supported TEMPO is easily obtained in a one-step reductive amination
procedure starting from a commercially available aminopropyl-functionalized
silica. This supported catalyst mediates the Anelli oxidation of various
alcohols. The recyclability and stability of the applied silica-supported TEMPO
have been studied.
T. Fey, H. Fischer, S. Bachmann, K. Albert, C. Bolm, J. Org. Chem., 2001,
66, 8154-8159.

Imidazolium salts bearing TEMPO groups generate in situ Cu-NHC-TEMPO complexes
with commercially available copper powder. These easily available Cu-NHC-TEMPO
complexes are quite efficient catalysts for selective, aerobic oxidation of
primary alcohols into aldehydes in excellent yields.
X. Liu, Q. Xia, Y. Zhang, C. Chen, W. Chen, J. Org. Chem., 2013,
78, 8531-8536.

A convenient method enables the preparation of a silica gel supported
TEMPO catalyst. The catalyst prepared from [4-hydroxy-TEMPO + NaCl]/SiO2
was used for an aerobic oxidation of alcohols to carbonyls under mild reaction
conditions in the presence of Fe(NO3)3 • 9 H2O.
Alcohols were converted to the corresponding carbonyls in good to excellent
yields. After a simple filtration, the catalyst can be reused at least six times.
N. Tamura, T. Aoyama, T. Takido, M. Kodomari, Synlett, 2012, 23,
1397-1407.

An efficient oxidation of primary alcohols to the corresponding aldehydes can be
carried out at room temperature in DCM, using trichloroisocyanuric acid in the
presence of catalytic TEMPO: aliphatic, benzylic, and allylic alcohols, and
β-amino alcohols are rapidly oxidized without no overoxidation to carboxylic
acids. The slow oxidation of secondary carbinols makes the reaction highly
chemoselective.
L. De Luca, G. Giacomelli, A. Porcheddu, Org. Lett., 2001,
3, 3041-3043.

2-Iodoxybenzenesulfonic acid, which can be generated in situ from
2-iodobenzenesulfonic acid sodium salt, is a much more active catalyst than
modified IBXs for the oxidation of alcohols with Oxone. Highly efficient and
selective methods for the oxidation of alcohols to carbonyl compounds such as
aldehydes, carboxylic acids, and ketones were established.
M. Uyanik, M. Akakura, K. Ishihara, J. Am. Chem. Soc., 2009,
131, 251-262.

Swern oxidation using the volatile oxalyl chloride as an activator requires
reaction temperatures below -60 °C. 3,3-Dichloro-1,2-diphenylcyclopropene (DDC)
can be used as a new activator at −20 °C. This convenient new protocol offers
mild and fast reactions. Furthermore, the activator DDC is easy to handle, and
diphenylcyclopropenone can be recovered quantitively.
T. Guo, Y. Gao, Z. Li, J. Liu, K. Guo, Synlett, 2019,
30,
329-332.

A mild and efficient alternative procedure for a quantitative conversion of
alcohols into the corresponding carbonyl compounds uses dimethyl sulfoxide
(DMSO), activated by 2,4,6-trichloro[1,3,5]-triazine (cyanuric chloride, TCT) instead of the toxic and moisture sensitive oxalyl chloride
under Swern conditions.
L. De Luca, G. Giacomelli, A. Porcheddu, J. Org. Chem., 2001,
66, 7907-7909.

In the presence of dimethyl sulfoxide, the Burgess reagent efficiently and
rapidly facilitates the oxidation of a broad range of primary and secondary
alcohols to their corresponding aldehydes and ketones in excellent yields and
under mild conditions. This oxidation can be combined with Wittig olefinations. A
mechanism similar to those described for the Pfitzner-Moffatt and Swern
oxidations is proposed.
P. R. Sultane, C. W. Bielawski, J. Org. Chem.,
2017, 82, 1046-1052.

A mild and efficient oxidation of alcohols with o-iodoxybenzoic acid
(IBX) is catalyzed by β-cyclodextrin in a water/acetone mixture (86:14).
Various alcohols were oxidized at room temperature in
excellent yields.
K. Surendra, N. Srilakshmi Krishnaveni, M. Arjun Reddy, Y. V. D. Nageswar, K.
Rama Rao, J. Org. Chem., 2003,
68, 2058-2059.

A novel, metal-free oxidation system for the catalytic synthesis of aldehydes
and ketones using TEMPO and a quarternary ammonium salt as catalysts and Oxone
as oxidant proved especially successful for the synthesis of ketones. The mild
conditions tolerate even sensitive silyl protective groups which can otherwise
be cleaved in the presence of Oxone.
C. Bolm, A. S. Magnus, J. P. Hildebrand, Org. Lett., 2000, 2,
1173-1175.

A highly efficient 2,2,6,6-tetramethylpiperidin-1-yloxy (TEMPO) catalyzed
reaction using recyclable 1-chloro-1,2-benziodoxol-3(1H)-one as the
terminal oxidant allows the conversion of various alcohols to their
corresponding carbonyl compounds in high to excellent yields at room temperature
in ethyl acetate, which is an environmentally friendly organic solvent.
X.-Q. Li, C. Zhang, Synthesis, 2009,
1163-1169.

A nitroxyl-radical-catalyzed oxidation using diisopropyl azodicarboxylate
(DIAD) allows the conversion of various primary and secondary alcohols to their
corresponding aldehydes and ketones without overoxidation to carboxylic acids.
1,2-Diols are oxidized to hydroxyl ketones or diketones depending on the amount
of DIAD used.
M. Hayashi, M. Shibuay, Y. Iwabuchi, J. Org. Chem., 2012,
77, 3005-3009.

Tetrapropylammonium perruthenate enables oxidations of a wide range of molecules
including examples of both double oxidations and selective oxidations.
Mechanistic studies and general experimental procedures are reported. In
addition several interesting developments in the chemistry of this reagent are
outlined: heteroatom oxidation, cleavage reactions and use in sequential
reaction processes.
S. B. Ley, J. Norman, W. P. Griffith, S. P. Marsden, Synthesis, 1994,
639-666.

he mild instability of the Ley-Griffith catalyst (TPAP) creates preparation,
storage, and reaction reproducibility issues, due to unpreventable slow
decomposition. A set of readily synthesized, bench stable, phosphonium
perruthenates (ATP3 and MTP3) mirror the reactivity of TPAP, but avoid storage
decomposition issues.
P. W. Moore, C. D. G. Read, P. V. Bernhardt, C. M. Williams, Chem. Eur. J., 2018, 24, 4556-4561.

CeBr3/H2O2 is a very efficient system for
the green oxidation of secondary and benzylic alcohols to carbonyls. The
mechanism involves the generation of a reactive brominating species (RBS) with
high oxidation selectivity of secondary over primary alcohols.
C. He, F. Ma, W. Zhang, R. Tong, Org. Lett.,
2022, 24, 3499-3503.

The use of an ortho-naphthoquinone catalyst enables a biomimetic
alcohol dehydrogenase (ADH)-like oxidation protocol as green alternative to
existing stoichiometric and metal-catalyzed alcohol oxidation reactions. The
developed organocatalytic aerobic oxidation protocol proceeds through an
intramolecular 1,5-hydrogen atom transfer of naphthalene alkoxide intermediates.
J. Baek, T. Si, H. Y. Kim, K. Oh, Org. Lett.,
2022, 24, 4982-4986.

The use of DMSO in the presence of H2SO4 enables an
efficient metal-free oxidation of benzylic alcohols to aromatic aldehydes in
excellent yields. This oxidation proceeds in short reaction time without side
products.
E. Sheikhi, M. Adib, M. A. Karajabad, S. J. A. Gohari,
Synlett, 2018, 29, 974-978.

The use of low loadings of a silver NHC catalysts enables a mild, selective
oxidation of alcohols to aldehydes or carboxylic acids in the presence of BnMe3NOH
or KOH under dry air in excellent yield. The catalytic system can also be used
for a one-pot synthesis of imines in excellent yield.
L. Han, P. Xing, B. Jiang, Org. Lett., 2014,
16, 3428-3431.

A catecholaldimine ligand based nickel(II) complex showed excellent
catalytic activity for the conversion of aromatic and heterocyclic alcohols into
trans-cinnamonitriles in a one-pot manner via oxidative olefination in
the presence of KOH. A direct conversion of alcohols to aldehydes is also
reported.
V. Sharma, K. A. Chavan, G. Mali, D. Sarkar, P. Lama, M. Majumder, R. D.
Erande, R. K. Metre, J. Org. Chem., 2023, 88,
7448-7453.

Copper N-heterocyclic carbene complexes serve as catalysts for both aerobic
oxidation of alcohols to aldehydes and reduction of imines to amines. A one-pot
tandem synthetic strategy affords useful secondary amines from benzylic alcohols
and anilines via an oxidation-reduction strategy.
L.-W. Zhan, L. Han, P. Xing, B. Jiang, Org. Lett.,
2015,
17, 5990-5993.

N-functionalized amino acids serve as powerful N,O-bidentate ligands in an
aerobic copper/TEMPO-catalyzed system for the oxidation of benzylic alcohols in
water. Under optimized reaction conditions, a broad range of primary and
secondary benzylic alcohols have been efficiently converted into carbonyl
compounds in very good yields.
G. Zhang, J. Lei, X. Han, Y. Luan, C. Ding, S. Shan,
Synlett, 2015, 26, 779-784.

A combination of FeCl3, L-valine and TEMPO oxidizes a wide range of
primary/secondary benzyl, allylic, and heterocyclic alcohols to aldehydes and
ketones with good to excellent isolated yields in the presence of oxygen.
G. Zhang, S. Li, J. Lei, G. Zhang, X. Xie, C. Ding, R. Liu,
Synlett, 2016, 27, 956-960.

Eosin Y catalyzes an efficient photochemical aerobic oxidation of various
benzyl alcohols to the corresponding aldehydes or ketones in excellent yields
under mild reaction conditions using O2 as oxidant. The catalyst
system offers good functional-group tolerance and exquisite chemoselectivity.
Z.-X. He, B. Yin, X.-H. Li, X.-L. Zhou, H.-N. Song, B.-B. Xu, F. Gao, J. Org. Chem., 2023, 88,
4765-4769.

Various imidazolium salts bearing hydrophilic groups afford water-soluble NHC
copper complexes. These copper complexes catalyze a selective oxidation of
benzyl alcohols to the corresponding aldehydes in water at room temperature
without the need for a base.
C. Chen, B. Liu, W. Chen, Synthesis, 2013, 45,
3387-3391.

A water-soluble Cp*Ir complex bearing a bipyridine-based functional ligand can
be used as catalyst for a dehydrogenative oxidation of various primary and
secondary alcohols to aldehydes and ketones, respectively without any oxidant.
The catalyst can be reused.
R. Kawahara, K.-i. Fujita, R. Yamaguchi, J. Am. Chem. Soc., 2012,
134, 3643-3646.

Urea-hydrogen peroxide in the presence of a catalytic amount of magnesium
bromide efficiently oxidizes primary and secondary
benzylic alcohols into the corresponding aromatic aldehydes and ketones.
H. J. Park, J. C. Lee, Synlett, 2009,
79-80.

TEMPO-derived reagents tagged with multiple perfluoroalkyl chains and
triazole moieties promote the oxidation of alcohols to aldehydes in organic
solvent/water mixtures with reaction rates comparable to homogeneous TEMPO
reagents, but can be easily recovered by liquid/emulsion filtration.
A. Gheorghe, T. Chinnusamy, E. Cuevas-Yañez, P. Hilgers, O. Reiser, Org. Lett.,
2008,
10, 4171-4174.

A rapid oxidation of primary and secondary alcohols using catalytic amounts of
TEMPO and Yb(OTf)3 in combination with a stoichiometric amount of
iodosylbenzene afforded carbonyl compounds in excellent yields without
over-oxidation. Oxidation of primary alcohols in the presence of secondary
alcohols proceeded with good selectivity.
J.-M. Vatèle, Synlett, 2006,
2055-2058.

Optimized selective aerobic oxidations in ionic liquids convert various
activated primary alcohols into their corresponding acids or aldehydes in good
to excellent yields. The newly developed catalytic systems could also be
recycled and reused for three runs without any significant loss of catalytic
activity.
N. Jiang, A. J. Ragauskas, J. Org. Chem.,
2007,
72, 7030-7033.

The combination of TEMPO and CAN can be used for the aerobic oxidation of
benzylic and allylic alcohols into their corresponding carbonyl compounds.
However, steric hindrance has been observed to impede the reaction with some
substituted allylic systems. The present method is superior to others
currently available due to its relatively short reaction times and excellent
yields.
S. S. Kim, H. C. Jung, Synthesis, 2003, 2135-2137.

The reaction of KBrO3 and NH2OH • HCl in situ generates NOx
and Br anion, which allows in the presence of 2,2,6,6-tetramethylpiperidine-N-oxide
(TEMPO) an activation of dioxygen to oxidize various benzylic alcohols
quantitatively to their corresponding carbonyl compounds under mild conditions.
G. Yang, W. Wang, W. Zhu, C. An, X. Gao, M. Song, Synlett, 2010,
437-440.

A highly efficient and mild procedure for the oxidation of different types of
alcohols uses TEMPO as catalyst, iodobenzene dichloride as stoichiometric
oxidant, and pyridine as base. Oxidation of 1,2-diols gives α-hydroxy ketones or
α-diketones depending on the amount of oxidant used. High yielding procedures
for the preparation of iodoarene dichlorides have been developed.
X.-F. Zhao, C. Zhang, Synthesis, 2007,
551-557.

Keggin-type heteropoly acids revealed high catalytic activity for swift and
selective oxidation of various hydroxy functionalities to the corresponding
carbonyl groups using ferric nitrate as an oxidant under mild and solvent-free
conditions.
H. Firouzabadi, N. Iranpoor, K. Amani, Synthesis, 2003, 408-412.

A new, green, mild and inexpensive system, I2-KI-K2CO3-H2O,
selectively oxidized alcohols to aldehydes and ketones under anaerobic condition
in water at 90 °C with excellent yields.
P. Gogoi, D. Konwar, Org. Biomol. Chem., 2005, 3, 3473-3475.

Pyridinium chlorochromate is a readily available, stable reagent, that oxidizes
a wide variety of alcohols to carbonyl compounds with high efficiency.
E. J. Corey, J. W. Suggs, Tetrahedron Lett.,
1975, 16, 2647-2650.

Permanganate supported on active manganese dioxide can
be used effectively for the oxidation of
arenes, alcohols and sulfides under heterogeneous
or solvent-free conditions.
A. Shaabania, P. Mirzaeia, S. Naderia, D. G. Leeb, Tetrahedron, 2004, 60, 11415-11420.

A heterogeneous palladium(II) acetate-pyridine complex supported by hydrotalcite catalyzes
the aerobic oxidation of a variety of primary and secondary alcohols into the corresponding aldehydes and ketones in high yields using atmospheric pressure of air as a sole oxidant under mild conditions
in toluene.
N. Kakiuchi, Y. Maeda, T. Nishimura, S. Uemura, J. Org. Chem., 2001,
66, 6620-6625.

Pd/C in aqueous alcohol with molecular oxygen, sodium borohydride,
and potassium carbonate efficiently oxidized benzylic and allylic alcohols.
Sodium borohydride allows a remarkable reactivation of active sites of the Pd
surface.
G. An, M. Lim, K.-S. Chun, H. Rhee, Synlett, 2007, 95-98.

A new, highly recoverable palladium-based catalyst for the aerobic oxidation
of alcohols combines an organic ligand and mesoporous channels that led to
enhanced activity, prevention of agglomeration and the generation of a
durable catalyst.
B. Karimi, S. Abedi, J. H. Clark, V. Budarin, Angew. Chem. Int. Ed., 2006, 45, 4776-4779.

A robust and effective Pd catalyst for the aerobic oxidation of various alcohols
has been discovered. Using a slightly higher concentration of acetic acid as
additive and extending the reaction times, the oxidation can be carried out
under ambient atmosphere of air.
D. R. Jensen, M. J. Schultz, J. A. Mueller, M. S. Sigman, Angew. Chem.
Int. Ed.,
2003, 42, 3810-3813.

A chemoselective and efficient procedure allows the conversion of benzylic
and allylic alcohols into the corresponding carbonyl compounds with sodium
nitrate as oxidant in the presence of 3-methylimidazolinium hydrogensulfate.
A. R. Hajipour, F. Rafiee, A. E. Ruoho, Synlett, 2007,
1118-1119.

A four-component system consisting of acetamido-TEMPO/Cu(ClO4)2/TMDP/DABCO
in DMSO allows an efficient room-temperature aerobic alcohol oxidation of
various alcohols into their corresponding aldehydes or ketones in good to
excellent yields. The catalytic system can be recycled.
N. Jiang, A. J. Ragauskas, J. Org. Chem., 2006, 71, 7087-7090.

The system Cu(ClO4)2/acetamido-TEMPO/DMAP catalyses
the room-temperature aerobic oxidation of primary alcohols to aldehydes
in the
ionic liquid [bmpy]PF6. The catalysts can be recycled and
reused.
N. Jiang, A. J. Ragauskas, Org. Lett., 2005, 7, 3689-3692.

The oxidation of primary and secondary alcohols by sodium percarbonate in the presence of catalytic
amounts of both molybdenyl acetylacetonate and Adogen 464 gave fair to high yields
of the corresponding carbonyl compounds.
S. Maignien, S. Aït-Mohand, J. Muzart, Synlett, 1996, 439-440.

[dibmim][BF4] can be used for the oxidation
of alcohols to carbonyl compounds. This oxidizing agent offers a high
degree of selectivity for the oxidation of primary alcohols to carbonyl
compounds without oxidation to carboxylic acids in ionic liquids. [dibmim][BF4]
can be reused after oxidation with peracetic acid.
W. Qian, E. Jin, W. Bao, Y. Zhang, Angew. Chem. Int. Ed., 2005,
44, 952-955.

An efficient oxidant-free oxidation for a wide range of alcohols was
achieved by a recyclable ruthenium catalyst, which was prepared from readily
available reagents through nanoparticle generation and gelation.
W.-H. Kim, I. S. Park, J. Park, Org. Lett.,
2006, 8, 2543-2545.

Adsorbed [RuCl2(p-cymene)]2
on activated carbon is an efficient, environmentally attractive and highly
selective catalyst for use in aerobic oxidations, hydrolytic oxidations and
dehydrations. The heterogeneous catalyst was recovered quantitatively by simple
filtration and could be reused with minimal loss of activity.
E. Choi, C. Lee, Y. Na, S. Chang, Org. Lett., 2002, 4,
2369-2371.

Benzyl alcohols and benzyl TBDMS ethers were
efficiently oxidized to the corresponding carbonyl compounds in high yield with
periodic acid catalyzed by CrO3 at low temperature (-78 °C). The oxidation
procedure was highly functional group tolerant and very selective for the TBDMS
group over the TBDPS group.
S. Zhang, L. Xu, M. L. Trudell, Synthesis, 2005,
1757-1760.

1 mol-% TEMPO and a catalytic
amount of 1,3-dibromo-5,5-dimethylhydantoin and NaNO2 is a highly efficient catalytic system for
the aerobic oxidations of benzylic alcohols in water.
R. Liu, C. Dong, X. Liang, X. Wang, X. Hu, J. Org. Chem., 2005, 70, 239-244.

A simple and mild TEMPO-CuCl catalyzed aerobic oxidation of primary and
secondary alcohols in ionic liquid [bmim][PF6] gave the
corresponding aldehydes and ketones with no trace of overoxidation to carboxylic
acids. The product can be isolated by a simple extraction with organic solvent,
and the ionic liquid can be recycled or reused.
I. A. Ansari, R. Gree, Org. Lett., 2002, 4, 1507-1509.

Benzylic ethers are oxidatively cleaved by
4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate in wet
MeCN at room temperature to give the corresponding aromatic aldehydes and
alcohols in high yield. Primary and secondary alkyl alcohols are further
oxidized to give carboxylic acids and ketones, respectively.
P. P. Pradhan, J. M. Bobbitt, W. F. Bailey, J. Org. Chem., 2009,
74, 9501-9504.

A highly convenient organocatalytic method for the mono-oxidation of
unprotected glycosides relies on the chemoselective properties of TEMPO in
combination with trichloroisocyanuric acid under very mild, basic conditions.
The resulting dialdo-glycosides are efficiently purified with the use of
solid-phase imine capture.
M. Angelin, M. Hermansson, H. Dong, O. Ramström, Eur. J. Org. Chem., 2006,
4323-4326.

Iodine was compared to other positive halogens as terminal oxidant in
chemoselective oxidations of alcohols using catalytic TEMPO and was shown to be
superior in cases of electron-rich and heteroaromatic benzylic alcohols.
R. A. Miller, R. S. Hoerrner, Org. Lett., 2003, 5,
285-287.

Oxidation of alcohols to aldehydes and ketones were performed under
atmospheric oxygen with a catalytic amount of V2O5 in
toluene at 100°C. Secondary alcohols can be chemoselectively converted into
ketones in the presence of primary hydroxy groups.
S. Velusamy, T. Punniyamurthy, Org. Lett., 2004, 6,
217-219.

The use of PhI(OAc)2 in dichloromethane enables a clean oxidative
cleavage of 1,2-diols to aldehydes. In the presence of OsO4 as
catalyst, NMO and 2,6-lutidine, olefinic bonds can be cleaved in acetone/water
to yield the corresponding carbonyl compounds.
K. C. Nicolaou, V. A. Adsool, C. R. H. Hale, Org. Lett., 2010,
12, 1552-1555.

A sequential one-pot synthesis for the oxidation of primary and secondary
tert-butyldimethylsilyl (TBDMS) ethers, using the presence of PhIO or
PhI(OAc)2 and catalytic amounts of metal triflates and TEMPO in THF
or acetonitrile tolerates acid-sensitive protecting groups and leaves tert-butyldiphenylsilyl
ethers and phenolic TBDMS groups untouched.
B. Barnych, J.-M. Vatèle, Synlett, 2011,
2048-2052.



