Categories: C=O Bond Formation > Synthesis of ketones >
Synthesis of ketones by oxidation of alcohols
Name Reactions
Recent Literature

CeBr3/H2O2 is a very efficient system for
the green oxidation of secondary and benzylic alcohols to carbonyls. The
mechanism involves the generation of a reactive brominating species (RBS) with
high oxidation selectivity of secondary over primary alcohols.
C. He, F. Ma, W. Zhang, R. Tong, Org. Lett.,
2022, 24, 3499-3503.

A ternary hybrid catalyst system comprising a photoredox catalyst, a
thiophosphate organocatalyst, and a nickel catalyst enables an acceptorless dehydrogenation of aliphatic secondary
alcohols to ketones under visible light irradiation at room temperature in high yield without producing side products (except H2 gas).
H. Fuse, H. Mitsunuma, M. Kanai, J. Am. Chem. Soc.,
2020, 142, 4493-4499.

2-Iodoxybenzenesulfonic acid, which can be generated in situ from
2-iodobenzenesulfonic acid sodium salt, is a much more active catalyst than
modified IBXs for the oxidation of alcohols with Oxone. Highly efficient and
selective methods for the oxidation of alcohols to carbonyl compounds such as
aldehydes, carboxylic acids, and ketones were established.
M. Uyanik, M. Akakura, K. Ishihara, J. Am. Chem. Soc., 2009,
131, 251-262.

An efficient bismuth tribromide catalyzed oxidation of various alcohols with
aqueous hydrogen peroxide provides carbonyl compounds in good yields.
M.-k. Han, S. Kim, S. T. Kim, J. C. Lee,
Synlett, 2015, 26, 2434-2436.

Oxidation of primary and secondary alcohols, using catalytic amounts of TEMPO
and tetra-n-butylammonium bromide in combination with periodic acid and
wet alumina in dichloromethane is compatible with a broad range of functional
groups and acid-sensitive protecting groups. The system also enables a
chemoselective oxidation of secondary alcohols in the presence of primary
alcohols.
M. Attoui, J.-M. Vatèle,
Synlett, 2014, 25, 2923-2927.
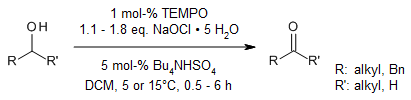
Sodium hypochlorite pentahydrate crystals with very low NaOH and NaCl contents
oxidize primary and secondary alcohols to the corresponding aldehydes and
ketones in the presence of TEMPO/Bu4NHSO4 without pH
adjustment. This new oxidation method is also applicable to sterically hindered
secondary alcohols.
T. Okada, T. Asawa, Y. Sugiyama, M. Kirihara, T. Iwai, Y. Kimura, Synlett, 2014, 25,
596-598.

Silica-supported TEMPO is easily obtained in a one-step reductive amination
procedure starting from a commercially available aminopropyl-functionalized
silica. This supported catalyst mediates the Anelli oxidation of various
alcohols. The recyclability and stability of the applied silica-supported TEMPO
have been studied.
T. Fey, H. Fischer, S. Bachmann, K. Albert, C. Bolm, J. Org. Chem., 2001,
66, 8154-8159.

The combination of Fe(NO3)3·9H2O and
9-azabicyclo[3.3.1]nonan-N-oxyl enables an efficient aerobic oxidation of
a broad range of primary and secondary alcohols to the corresponding aldehydes
and ketones at room temperature with ambient air as the oxidant.
L. Wang, S. Shang, G. Li, L. Ren, Y. Lv, S. Gao, J. Org. Chem.,
2016,
81, 2189-2193.

Cu/TEMPO catalyst systems show reduced reactivity in aerobic oxidation of
aliphatic and secondary alcohols. A catalyst system consisting of (MeObpy)CuOTf
and ABNO mediates aerobic oxidation of primary, secondary allylic, benzylic, and
aliphatic alcohols with nearly equal efficiency. The catalyst exhibits broad
functional group compatibility, and most reactions are complete within 1 h at
room temperature using ambient air as oxidant.
J. E. Steves, S. S. Stahl, J. Am. Chem. Soc., 2013,
135, 15742-15745.

8-Azabicyclo[3.2.1]octan-8-ol (ABOOL) and 7-azabicyclo[2.2.1]heptan-7-ol (ABHOL)
feature small bicyclic backbones and are known to be stable. These
hydroxylamines can efficiently catalyze the oxidation of various secondary
alcohols to their corresponding ketones using molecular oxygen in ambient air as
the terminal oxidant and copper cocatalysts at room temperature.
M. Toda, Y. Sasano, M. Takahashi, S. Fujiki, K. Kasabata, T. Ono, K. Sato, Y.
Kashiwagi, Y. Iwabuchi, J. Org. Chem., 2023, 88,
1434-1444.

A stable nitroxyl radical class of catalysts, 2-azaadamantane N-oxyl (AZADO) and
1-Me-AZADO, exhibit superior catalytic proficiency to TEMPO, converting various
sterically hindered alcohols to the corresponding carbonyl compounds in
excellent yields.
M. Shibuya, M. Tomizawa, I. Suzuki, Y. Iwabuchi, J. Am. Chem. Soc., 2006,
128, 8412-8413.

An efficient oxidation of primary alcohols to the corresponding aldehydes can be
carried out at room temperature in DCM, using trichloroisocyanuric acid in the
presence of catalytic TEMPO: aliphatic, benzylic, and allylic alcohols, and
β-amino alcohols are rapidly oxidized without no overoxidation to carboxylic
acids. The slow oxidation of secondary carbinols makes the reaction highly
chemoselective.
L. De Luca, G. Giacomelli, A. Porcheddu, Org. Lett., 2001,
3, 3041-3043.

The combination of N-hydroxyphthalimide (NHPI), a Co species, and
optionally a small amount of a (per)benzoic acid catalyzes highly efficient
oxidations of alcohols with oxygen. Primary alcohols are rapidly oxidized to the
corresponding carboxylic acids, terminal vic-diols give one carbon less
carboxylic acids, while internal vic-diols were selectively oxidized to
1,2-diketones.
T. Iwahama, Y. Yoshino, T. Keitoku, S. Sakaguchi, Y. Ishii, J. Org. Chem., 2000,
65, 6502-6507.

A recyclable, polymeric phosphotungstate catalyst bearing a poly(ethylene
oxide-pyridinium) matrix efficiently promoted oxidation of various secondary
alcohols, including highly sterically demanding neopentyl alcohols, with
hydrogen peroxide, to afford the corresponding carbonyl compounds in up to
quantitative yield. The chemoselective oxidation in the presence of primary
alcohols was achieved.
Y. M. A. Yamada, C. K. Jin, Y. Uozumi, Org. Lett., 2010,
12, 4540-4543.

A water-soluble Cp*Ir complex bearing a bipyridine-based functional ligand can
be used as catalyst for a dehydrogenative oxidation of various primary and
secondary alcohols to aldehydes and ketones, respectively without any oxidant.
The catalyst can be reused.
R. Kawahara, K.-i. Fujita, R. Yamaguchi, J. Am. Chem. Soc., 2012,
134, 3643-3646.

The choline- and peroxydisulfate-based environmentally benign biodegradable
oxidizing task-specific ionic liquid (TSIL) choline peroxydisulfate (ChPS) was
synthesized and characterized. This reagent enables a selective oxidation of
alcohols to aldehydes/ketones in very good yields and short reaction time under
solvent-free mild reaction conditions without overoxidation to acid.
B. L. Gadilohar, H. S. Kumbhar, G. S. Shankarling, Ind. Eng. Chem. Res., 2014,
53, 19010-19018.

In the presence of dimethyl sulfoxide, the Burgess reagent efficiently and
rapidly facilitates the oxidation of a broad range of primary and secondary
alcohols to their corresponding aldehydes and ketones in excellent yields and
under mild conditions. This oxidation can be combined with Wittig olefinations. A
mechanism similar to those described for the Pfitzner-Moffatt and Swern
oxidations is proposed.
P. R. Sultane, C. W. Bielawski, J. Org. Chem.,
2017, 82, 1046-1052.

Swern oxidation using the volatile oxalyl chloride as an activator requires
reaction temperatures below -60 °C. 3,3-Dichloro-1,2-diphenylcyclopropene (DDC)
can be used as a new activator at −20 °C. This convenient new protocol offers
mild and fast reactions. Furthermore, the activator DDC is easy to handle, and
diphenylcyclopropenone can be recovered quantitively.
T. Guo, Y. Gao, Z. Li, J. Liu, K. Guo, Synlett, 2019,
30,
329-332.

A mild and efficient alternative procedure for a quantitative conversion of
alcohols into the corresponding carbonyl compounds uses dimethyl sulfoxide
(DMSO), activated by 2,4,6-trichloro[1,3,5]-triazine (cyanuric chloride, TCT) instead of the toxic and moisture sensitive oxalyl chloride
under Swern conditions.
L. De Luca, G. Giacomelli, A. Porcheddu, J. Org. Chem., 2001,
66, 7907-7909.

A mild and efficient oxidation of alcohols with o-iodoxybenzoic acid
(IBX) is catalyzed by β-cyclodextrin in a water/acetone mixture (86:14).
Various alcohols were oxidized at room temperature in
excellent yields.
K. Surendra, N. Srilakshmi Krishnaveni, M. Arjun Reddy, Y. V. D. Nageswar, K.
Rama Rao, J. Org. Chem., 2003,
68, 2058-2059.

A convenient method enables the preparation of a silica gel supported
TEMPO catalyst. The catalyst prepared from [4-hydroxy-TEMPO + NaCl]/SiO2
was used for an aerobic oxidation of alcohols to carbonyls under mild reaction
conditions in the presence of Fe(NO3)3 • 9 H2O.
Alcohols were converted to the corresponding carbonyls in good to excellent
yields. After a simple filtration, the catalyst can be reused at least six times.
N. Tamura, T. Aoyama, T. Takido, M. Kodomari, Synlett, 2012, 23,
1397-1407.

A nitroxyl-radical-catalyzed oxidation using diisopropyl azodicarboxylate
(DIAD) allows the conversion of various primary and secondary alcohols to their
corresponding aldehydes and ketones without overoxidation to carboxylic acids.
1,2-Diols are oxidized to hydroxyl ketones or diketones depending on the amount
of DIAD used.
M. Hayashi, M. Shibuay, Y. Iwabuchi, J. Org. Chem., 2012,
77, 3005-3009.

The combination of TEMPO and CAN can be used for the aerobic oxidation of
benzylic and allylic alcohols into their corresponding carbonyl compounds.
However, steric hindrance has been observed to impede the reaction with some
substituted allylic systems. The present method is superior to others
currently available due to its relatively short reaction times and excellent
yields.
S. S. Kim, H. C. Jung, Synthesis, 2003, 2135-2137.

A rapid oxidation of primary and secondary alcohols using catalytic amounts of
TEMPO and Yb(OTf)3 in combination with a stoichiometric amount of
iodosylbenzene afforded carbonyl compounds in excellent yields without
over-oxidation. Oxidation of primary alcohols in the presence of secondary
alcohols proceeded with good selectivity.
J.-M. Vatèle, Synlett, 2006,
2055-2058.

A novel, metal-free oxidation system for the catalytic synthesis of aldehydes
and ketones using TEMPO and a quarternary ammonium salt as catalysts and Oxone
as oxidant proved especially successful for the synthesis of ketones. The mild
conditions tolerate even sensitive silyl protective groups which can otherwise
be cleaved in the presence of Oxone.
C. Bolm, A. S. Magnus, J. P. Hildebrand, Org. Lett., 2000, 2,
1173-1175.

Catalytic use of o-iodoxybenzoic acid (IBX) in the presence of Oxone as a
co-oxidant is demonstrated for the oxidation of primary and secondary alcohols.
In addition, the in situ oxidation
of 2-iodosobenzoic acid (IBA) and even commercially available 2-iodobenzoic acid
(2IBAcid) by Oxone to IBX allows the use of these less hazardous reagents, in
place of potentially explosive IBX, as catalytic oxidants.
A. P. Thottumkara, M. S. Bowsher, T. K. Vinod, Org. Lett., 2005, 7, 2933-2936.

A highly efficient 2,2,6,6-tetramethylpiperidin-1-yloxy (TEMPO) catalyzed
reaction using recyclable 1-chloro-1,2-benziodoxol-3(1H)-one as the
terminal oxidant allows the conversion of various alcohols to their
corresponding carbonyl compounds in high to excellent yields at room temperature
in ethyl acetate, which is an environmentally friendly organic solvent.
X.-Q. Li, C. Zhang, Synthesis, 2009,
1163-1169.

A new, green, mild and inexpensive system, I2-KI-K2CO3-H2O,
selectively oxidized alcohols to aldehydes and ketones under anaerobic condition
in water at 90 °C with excellent yields.
P. Gogoi, D. Konwar, Org. Biomol. Chem., 2005, 3, 3473-3475.

A simple, efficient, and high-yield procedure for the oxidative conversion of
alcohols to various types of esters and ketones was successfully carried out
with molecular iodine as the oxidant and potassium carbonate.
N. Mori, H. Togo, Tetrahedron, 2005,
61, 5915-5925.

A heterogeneous palladium(II) acetate-pyridine complex supported by hydrotalcite catalyzes
the aerobic oxidation of a variety of primary and secondary alcohols into the corresponding aldehydes and ketones in high yields using atmospheric pressure of air as a sole oxidant under mild conditions
in toluene.
N. Kakiuchi, Y. Maeda, T. Nishimura, S. Uemura, J. Org. Chem., 2001,
66, 6620-6625.

A chemoselective and efficient procedure allows the conversion of benzylic
and allylic alcohols into the corresponding carbonyl compounds with sodium
nitrate as oxidant in the presence of 3-methylimidazolinium hydrogensulfate.
A. R. Hajipour, F. Rafiee, A. E. Ruoho, Synlett, 2007,
1118-1119.

Keggin-type heteropoly acids revealed high catalytic activity for swift and
selective oxidation of various hydroxy functionalities to the corresponding
carbonyl groups using ferric nitrate as an oxidant under mild and solvent-free
conditions.
H. Firouzabadi, N. Iranpoor, K. Amani, Synthesis, 2003, 408-412.

A combination of FeCl3, L-valine and TEMPO oxidizes a wide range of
primary/secondary benzyl, allylic, and heterocyclic alcohols to aldehydes and
ketones with good to excellent isolated yields in the presence of oxygen.
G. Zhang, S. Li, J. Lei, G. Zhang, X. Xie, C. Ding, R. Liu,
Synlett, 2016, 27, 956-960.

Eosin Y catalyzes an efficient photochemical aerobic oxidation of various
benzyl alcohols to the corresponding aldehydes or ketones in excellent yields
under mild reaction conditions using O2 as oxidant. The catalyst
system offers good functional-group tolerance and exquisite chemoselectivity.
Z.-X. He, B. Yin, X.-H. Li, X.-L. Zhou, H.-N. Song, B.-B. Xu, F. Gao, J. Org. Chem., 2023, 88,
4765-4769.

Tetrapropylammonium perruthenate enables oxidations of a wide range of molecules
including examples of both double oxidations and selective oxidations.
Mechanistic studies and general experimental procedures are reported. In
addition several interesting developments in the chemistry of this reagent are
outlined: heteroatom oxidation, cleavage reactions and use in sequential
reaction processes.
S. B. Ley, J. Norman, W. P. Griffith, S. P. Marsden, Synthesis, 1994,
639-666.

he mild instability of the Ley-Griffith catalyst (TPAP) creates preparation,
storage, and reaction reproducibility issues, due to unpreventable slow
decomposition. A set of readily synthesized, bench stable, phosphonium
perruthenates (ATP3 and MTP3) mirror the reactivity of TPAP, but avoid storage
decomposition issues.
P. W. Moore, C. D. G. Read, P. V. Bernhardt, C. M. Williams, Chem. Eur. J., 2018, 24, 4556-4561.

Urea-hydrogen peroxide in the presence of a catalytic amount of magnesium
bromide efficiently oxidizes primary and secondary
benzylic alcohols into the corresponding aromatic aldehydes and ketones.
H. J. Park, J. C. Lee, Synlett, 2009,
79-80.

A highly efficient and mild procedure for the oxidation of different types of
alcohols uses TEMPO as catalyst, iodobenzene dichloride as stoichiometric
oxidant, and pyridine as base. Oxidation of 1,2-diols gives α-hydroxy ketones or
α-diketones depending on the amount of oxidant used. High yielding procedures
for the preparation of iodoarene dichlorides have been developed.
X.-F. Zhao, C. Zhang, Synthesis, 2007,
551-557.

The oxidation of primary and secondary alcohols by sodium percarbonate in the presence of catalytic
amounts of both molybdenyl acetylacetonate and Adogen 464 gave fair to high yields
of the corresponding carbonyl compounds.
S. Maignien, S. Aït-Mohand, J. Muzart, Synlett, 1996, 439-440.

A chemoselective oxidation of secondary alcohols with IBX/n-Bu4NBr
in CH2Cl2-H2O gave ketones in good yields and
allowed the oxidation of secondary hydroxyl group even in the presence of
primary hydroxyl groups.
C. Kuhakarn, K. Kittigowittana, M. Pohmakotr, V. Reutrakul, Tetrahedron, 2005,
61, 8995-9000.

Permanganate supported on active manganese dioxide can be used effectively
for the oxidation of arenes, alcohols and sulfides under heterogeneous or
solvent-free conditions.
A. Shaabania, P. Mirzaeia, S. Naderia, D. G. Leeb, Tetrahedron, 2004, 60, 11415-11420.

Benzyl alcohols and benzyl TBDMS ethers were
efficiently oxidized to the corresponding carbonyl compounds in high yield with
periodic acid catalyzed by CrO3 at low temperature (-78 °C). The oxidation
procedure was highly functional group tolerant and very selective for the TBDMS
group over the TBDPS group.
S. Zhang, L. Xu, M. L. Trudell, Synthesis, 2005,
1757-1760.

Pd/C in aqueous alcohol with molecular oxygen, sodium borohydride,
and potassium carbonate efficiently oxidized benzylic and allylic alcohols.
Sodium borohydride allows a remarkable reactivation of active sites of the Pd
surface.
G. An, M. Lim, K.-S. Chun, H. Rhee, Synlett, 2007, 95-98.

A new, highly recoverable palladium-based catalyst for the aerobic oxidation
of alcohols combines an organic ligand and mesoporous channels that led to
enhanced activity, prevention of agglomeration and the generation of a
durable catalyst.
B. Karimi, S. Abedi, J. H. Clark, V. Budarin, Angew. Chem. Int. Ed., 2006, 45, 4776-4779.

A robust and effective Pd catalyst for the aerobic oxidation of various alcohols
has been discovered. Using a slightly higher concentration of acetic acid as
additive and extending the reaction times, the oxidation can be carried out
under ambient atmosphere of air.
D. R. Jensen, M. J. Schultz, J. A. Mueller, M. S. Sigman, Angew. Chem.
Int. Ed.,
2003, 42, 3810-3813.

[dibmim][BF4] can be used for the oxidation
of alcohols to carbonyl compounds. This oxidizing agent offers a high
degree of selectivity for the oxidation of primary alcohols to carbonyl
compounds without oxidation to carboxylic acids in ionic liquids. [dibmim][BF4]
can be reused after oxidation with peracetic acid.
W. Qian, E. Jin, W. Bao, Y. Zhang, Angew. Chem. Int. Ed., 2005,
44, 952-955.

ReOCl3(PPh3)2 catalyzes a rapid oxidation of secondary alcohols by
DMSO in the presence of ethylene glycol and refluxing toluene to provide the corresponding
ketals in very good yields. Methyl sulfide and
water as byproducts of the reaction are easily removed.
J. B. Arterburn, M. C. Perry,
Org. Lett., 1999, 1, 769-771.

A practical aerobic oxidation of propargylic alcohols using Fe(NO3)3•9H2O,
TEMPO and sodium chloride in toluene at room temperature allows the conversion
of propargylic alcohols to α,β-unsaturated alkynals or alkynones in good to
excellent yields. This protocol can also be applied in industrial-scale
production.
J. Liu, X. Xie, S. Ma, Synthesis, 2012, 44,
1569-1576.

A highly efficient oxidation of propargylic alcohols to ynones is catalyzed by
copper nanoparticles (Cu Nps) with TBHP or air as oxidants. With bipyridine as
the ligand, the reaction was accelerated significantly and led in good to
excellent yields to a variety of propargylic alcohols.
C. Han, M. Yu, W. Sun, Y. Yao, Synlett, 2011,
2363-2368.

N-Iodosuccinimide (NIS) mediates a convenient and mild oxidation of
propargyl alcohols for the construction of ynones in good yields.
H. Qi, S. Xu, R. Zhao, S. Chen, J. Org. Chem., 2022, 87,
13726-13733.

A practical and environmentally friendly method for the
oxidative rearrangement of five- and six-membered cyclic tertiary allylic
alcohols to α,β-unsaturated β-disubstituted ketones by IBX in DMSO
is described. Several conventional protecting groups (e.g., Ac, MOM, and TBDPS)
are tolerated.
M. Shibuya, S. Ito, M. Takahashi, Y. Iwabuchi, Org. Lett., 2004, 6, 4303-4306.

An efficient oxidant-free oxidation for a wide range of alcohols was
achieved by a recyclable ruthenium catalyst, which was prepared from readily
available reagents through nanoparticle generation and gelation.
W.-H. Kim, I. S. Park, J. Park, Org. Lett.,
2006, 8, 2543-2545.

Adsorbed [RuCl2(p-cymene)]2
on activated carbon is an efficient, environmentally attractive and highly
selective catalyst for use in aerobic oxidations, hydrolytic oxidations and
dehydrations. The heterogeneous catalyst was recovered quantitatively by simple
filtration and could be reused with minimal loss of activity.
E. Choi, C. Lee, Y. Na, S. Chang, Org. Lett., 2002, 4,
2369-2371.

A simple and mild TEMPO-CuCl catalyzed aerobic oxidation of primary and
secondary alcohols in ionic liquid [bmim][PF6] gave the
corresponding aldehydes and ketones with no trace of overoxidation to carboxylic
acids. The product can be isolated by a simple extraction with organic solvent,
and the ionic liquid can be recycled or reused.
I. A. Ansari, R. Gree, Org. Lett., 2002, 4, 1507-1509.

Oxidation of alcohols to aldehydes and ketones were performed under
atmospheric oxygen with a catalytic amount of V2O5 in
toluene at 100°C. Secondary alcohols can be chemoselectively converted into
ketones in the presence of primary hydroxy groups.
S. Velusamy, T. Punniyamurthy, Org. Lett., 2004, 6,
217-219.

Benzylic ethers are oxidatively cleaved by
4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate in wet
MeCN at room temperature to give the corresponding aromatic aldehydes and
alcohols in high yield. Primary and secondary alkyl alcohols are further
oxidized to give carboxylic acids and ketones, respectively.
P. P. Pradhan, J. M. Bobbitt, W. F. Bailey, J. Org. Chem., 2009,
74, 9501-9504.

Trihaloacetic acids can been converted to trichloromethyl and tribromomethyl ketones in good
yield by a catalyzed reaction with aldehydes followed by oxidation. A coupling of organozinc intermediates with trichloroacetyl chloride
gives trichloromethyl ketones.
E. J. Corey, J. O. Link, Y. Shao, Tetrahedron Lett., 1992,
33, 3435-3438.

A green, practical, convenient, and cheap copper-catalyzed oxidative coupling of
aromatic alcohols and acetonitrile to β-ketonitriles involves a C-C coupling
with loss of two hydrogen atoms from the corresponding two carbons, using oxygen
as the terminal oxidant.
J. Shen, D. Yang, Y. Liu, S. Qin, J. Zhang, J. Sun, C. Liu, C. Liu, X. Zhao, C.
Chu, R. Liu, Org. Lett., 2014,
16, 350-353.

Formation of a bromo radical through the oxidation of bromide under mild
conditions enables an oxidative debenzylation of N-benzyl amides and O-benzyl
ethers to provide the corresponding amides and carbonyl compounds in high yields.
K. Moriyama, Y. Nakamura, H. Togo, Org. Lett., 2014,
16, 3812-3815.

A sequential one-pot synthesis for the oxidation of primary and secondary
tert-butyldimethylsilyl (TBDMS) ethers, using the presence of PhIO or
PhI(OAc)2 and catalytic amounts of metal triflates and TEMPO in THF
or acetonitrile tolerates acid-sensitive protecting groups and leaves tert-butyldiphenylsilyl
ethers and phenolic TBDMS groups untouched.
B. Barnych, J.-M. Vatèle, Synlett, 2011,
2048-2052.

A direct retro-Barbier fragmentation of cyclic tertiary alcohols proceeds under
mild conditions in the presence of bromine and potassium carbonate in chloroform
to provide bromoketones in high yields.
W.-C. Zhang, C.-J. Li, J. Org. Chem., 2000,
65, 5831-5833.

A metal-free ring opening/halogenation of cycloalkanols, which combines both PPO/TBAX
oxidant system and blue LEDs irradiation, provides diverse γ, δ, and even more
remotely halogenated ketones in good yields under mild conditions.
R. Zhao, Y. Yao, D. Zhu, D. Chang, Y. Liu, L. Shi, Org. Lett.,
2018, 20, 1228-1231.



