Categories: C=S Bond Formation >
Synthesis of thioamides
Related |
|
Name Reactions
Recent Literature

Aliphatic and aromatic nitriles react with thioacetic acid in the presence of
calcium hydride to give the corresponding thioamides in good to excellent yields.
Haloaryl nitriles do not undergo SNAr reactions under the described conditions.
K. A. Mahammed, V. P. Jayashankara, N. P. Rai, K. M. Raju, P. N. Arunachalam, Synlett, 2009,
2338-2340.

A simple, efficient, and new method has been developed for the synthesis of
thioamides from nitriles. The reaction of a variety of aromatic and
aliphatic nitriles in the presence of phosphorus pentasulfide afforded the
corresponding thioamides in high yields. This method is easy, rapid, and
high-yielding for the synthesis of thioamides from nitriles.
B. Kaboudin, D. Elhamifar, Synthesis, 2006, 224-226.

Water mediates a greener and mild synthesis of thioamides with no input
energy, additives, or catalysts. The presented protocol enables the conversion
of readily available starting materials and the use of different array amines,
and can easily be scaled-up.
A. Gupta, J. K. Vankar, J. P. Jadav, G. N. Gururaja, J. Org. Chem., 2022, 87,
2410-2420.

The reaction of various aromatic and aliphatic amides in the presence of
ammonium phosphorodithioate as an efficient thionation reagent proceeded
effectively to afford the corresponding thioamides in high yields. This method
is easy, rapid, and high-yielding for the synthesis of thioamides from amides
using an easily handled reagent.
B. Kaboudin, L. Malekzadeh, Synlett, 2011,
2807-2810.

An expeditious, solvent-free, and high yield conversion of ketones, flavones,
isoflavones, lactones, amides, and esters to the corresponding thio
analogues is described utilizing Lawesson's reagent in a process that
circumvents the use of dry solvents and excess of the reagent.
R. S. Varma, D. Kumar, Org. Lett., 1999, 1, 697-700.

A novel, simple and convenient thionation protocol for carbonyl compounds with
the system PSCl3/H2O/Et3N allows a clean,
rapid, and efficient synthesis of a variety of thiocarbonyl compounds such as
thioamides, thiolactams, thioketones, thioxanthones and thioacridone under
solventless condition with microwave irradiation.
U. Pathak, L. K. Pandey, R. Tank, J. Org. Chem., 2008,
73, 2890-2893.

Thionation of amides, 1,4-diketones, N-(2-oxoalkyl)amides, and N,N'-acylhydrazines
with the use of a fluorous Lawesson's reagent leads to thioamides, thiophenes,
1,3-thiazoles, and 1,3,4-thiadiazoles in high yields. The isolation of the final products is achieved in most cases by a
simple filtration.
Z. Kaleta, B. T Makowski, T. Soos, R. Dembinski, Org. Lett.,
2006, 8, 1625-1628.

A microwave-enhanced Kindler thioamide synthesis is
introduced. The three-component condensations of aldehydes, amines, and
elemental sulfur were carried out using 1-methyl-2-pyrrolidone (NMP) as solvent
employing microwave flash heating at 110-180°C for 2-20 min. A simple workup
protocol allows the isolation of synthetically valuable thioamide building blocks in good yield and purity.
O. I. Zubruyev, N. Stiasni, C. O. Kappe, J. Comb. Chem., 2003, 5, 145-148.

A nucleophilic activation of elemental sulfur by thiols enables a mild and
chemoselective thioacylation of amines with α-keto acids and elemental sulfur.
The reaction tolerates a broad range of functional groups, including unprotected
hydroxyl, carboxyl, amide, sulfide, and tertiary amine moieties.
M. Saito, S. Murakami, T. Nanjo, Y. Kobayashi, Y. Takemoto, J. Am. Chem. Soc.,
2020, 142, 8130-8135.

A convenient method for the synthesis of aryl thioamides from aryl aldehydes and
tetramethylthiuram disulfide (TMTD) in the presence of CuI and di-tert-butyl
peroxide (DTBP) avoids the use of a sulfurating reagent. The protocol offers
broad substrate scope, very good yields, operability and uses commercially
available and inexpensive raw materials.
M.-T. Zeng, M. Wang, H.-Y. Peng, Y. Cheng, Z.-B. Dong, Synthesis, 2018, 50,
644-650.

In an efficient aqueous synthesis of thioamides from aldehydes and N-substituted
formamides, sodium sulfide serves as the sulfur source in water, which makes
this method very practical and efficient. Furthermore, late-stage modifications
of bioactive molecules are demonstrated.
J. Wei, Y. Li, X. Jiang, Org. Lett., 2016, 18,
340-343.

Base-controlled three component reactions of styrenes, amines, and sulfur
provide thioamides. Depending on the base, 2-phenylethanethioamides and
benzothioamides were obtained selectively.
P. Zhang, W. Chen, M. Liu, H. Wu, J. Org. Chem., 2018, 83,
14269-14276.

A decarboxylative strategy for the synthesis of thioamides involves a
three-component reaction of arylacetic or cinnamic acids, amines and elemental
sulfur powder, without the need of a transition metal and an external oxidant.
T. Guntreddi, R. Vanjari, K. N. Singh, Org. Lett., 2014,
16, 3624-3625.

T. Guntreddi, R. Vanjari, K. N. Singh, Org. Lett., 2014,
16, 3624-3625.

A three-component reaction of alkynes, elemental sulfur, and aliphatic amines
allows a general, straightforward, and atom-economical synthesis of thioamides.
T. B. Nguyen, M. Q. Tran, L. Ermolenko, A. Al-Mourabit, Org. Lett., 2014,
16, 310-313.

A transition-metal-free cleavage of C-C triple bonds in aromatic alkynes in the
presence of S8 and amides furnishes aryl thioamides in good yields.
The method offers mild reaction conditions, as well as wide substrate scope that
is particularly compatible with some internal aromatic alkynes and acetamides.
K. Xu, Z. Li, F. Cheng, Z. Zuo, T. Wang, M. Wang, L. Liu, Org. Lett.,
2018, 20, 2228-2231.

In an environmentally friendly, atom-economical, and step-economical oxidation,
thiols are used as a synthon for the preparation of thioamides without the use
of exogenous sulfur reagents.
X. Wang, M. Ji, S. Lim, H.-Y. Jang, J. Org. Chem., 2014,
79, 7258-7260.
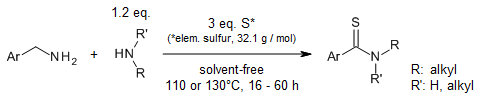
An efficient and selective multicomponent oxidative coupling of two different
aliphatic primary amines allows the synthesis of thioamides in the presence of
elemental sulfur under solvent-free conditions.
T. B. Nguyen, L. Ermolenko, A. Al-Mourabit, Org. Lett., 2012,
14, 4274-4277.

A sequential tandem approach using thiophosphoryl chloride as a dehydrating and
thionating agent enables the conversion of benzaldehyde oximes into primary
benzothioamides via benzonitriles.
L. K. Pandey, U. Pathak, S. Mathur, M. V. S. Suryanarayana, Synthesis, 2012, 44,
272-282.

A cleavage of the C=C bond in N,N-disubstituted enaminones in the
presence of elemental sulfur and DMAP provides N,N-disubstituted α-keto
thioamides without using any metal catalyst or additive.
L. Gan, Y. Gao, L. Wei, J.-P. Wan, J. Org. Chem., 2019, 84,
1064-1069.

The reaction of sulfoxonium ylides with primary or secondary amines afforded
α-ketothioamides in the presence of elemental sulfur, whereas α-ketoamides were
produced when I2 and TBHP were present. This simple, scalable
reaction proceeded well at room temperature, tolerated a range of functional
group, and generated the corresponding products in very good yields.
T. N. Chaubey, P. J. Borpatra, A. Sharma, S. K. Pandey, Org. Lett., 2022, 24,
8062-8066.

A metal-free oxidative-amidation strategy enables the synthesis of
α-ketothioamides and amides from α-azido ketones. The C-H bond thionation of
α-azido ketones with elemental sulfur could form α-ketothioacyl azide, which was
then nucleophilically attacked by amines, while amides could be formed with the
release of nitrogen gas and cyano anion in the presence of PhI(OAc)2.
P. Yu, Y. Wang, Z. Zeng, Y. Chen, J. Org. Chem., 2019, 84,
14883-14891.

Endothiopeptides can easily be obtained via Ugi reaction using thio acids as
acid components. If isonitriles with an acetal group are applied, the
endothiopeptides can directly be converted into thiazoles using TMSCl-NaI
under microwave irradiation.
U. Kazmaier, S. Ackermann, Org. Biomol. Chem., 2005, 3, 3184-3187.
