Categories: Synthesis of N-Heterocycles >
Synthesis of pyridines and related compounds
Name Reactions
Bohlmann-Rahtz Pyridine Synthesis

Hantzsch Dihydropyridine (Pyridine) Synthesis
Recent Literature

Addition of Grignard reagents to pyridine N-oxides in THF at room
temperature and subsequent treatment with acetic anhydride at 120°C afforded
2-substituted pyridines in good yields. By exchanging acetic anhydride for DMF
in the second step, 2-substituted pyridine N-oxides were obtained,
enabling the synthesis of 2,6-disubstituted pyridines.
H. Andersson, F. Almqvist, R. Olsson, Org. Lett., 2007,
9, 1335-1337.

The success of a one-step transformation of heterocyclic N-oxides to
2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles hinges on the
combination of copper catalysis and activation by lithium fluoride or magnesium
chloride. The utility for the scaffold decoration of a broad range of complex
N-heterocycles is exemplified by syntheses of new structural analogues of
several antimalarial, antimicrobial, and fungicidal agents.
O. V. Larionov, D. Stephens, A. Mfuh, G. Chavez, Org. Lett., 2014,
16, 864-867.

Cross-coupling of aryl bromides with 2-thienyl, 3-thienyl, 2-pyridyl, and
3-pyridyl aluminum reagents in the presence of Pd(OAc)2 and (o-tolyl)3P
provides useful biaryl building blocks. Additionally, the catalytic system was
also suited well for the coupling reaction of benzyl halides with pyridyl
aluminum reagents to afford a series of pyridylarylmethanes.
X. Chen, L. Zhou, Y. Li, T. Xie, S. Zhou, J. Org. Chem., 2014,
79, 230-239.

Mechanochemically activated magnesium(0) metal is a highly active mediator
for the direct C-4-H alkylation of pyridines with alkyl halides. The reaction
offers excellent regioselectivity and substrate scope, including those
containing reducible functionalities, free amines, and alcohols, as well as
biologically relevant molecules.
C. Wu, T. Ying, H. Fan, C. Hu, W. Su, J. Yu, Org. Lett., 2023, 25,
2531-2536.

A nickel-catalyzed reductive coupling of bromopyridines with tertiary alkyl
bromides provides alkylated pyridines bearing an all-carbon quaternary center.
This strategy features mild conditions, broad substrate scope, and high
functional group tolerance.
Q. Lin, H. Gong, F. Wu, Org. Lett., 2022, 24,
8996-9000.

A simple maleate-derived blocking group for pyridines enables exquisite
control for Minisci-type decarboxylative alkylation at C-4 that allows for
inexpensive access to a broad range of valuable building blocks. The method is
operationally simple and scalable, and is applied to access known structures in
a rapid and inexpensive fashion.
J. Choi, G. Laudadio, E. Godineau, P. S. Baran, J. Am. Chem. Soc.,
2021, 143, 11927-11933.

A photochemical cross-coupling between N-amidopyridinium salts and
various alkyl bromides under photocatalyst-free conditions provides various
C4-alkylated pyridines. The photochemical activity of electron donor-acceptor
(EDA) complexes between N-amidopyridinium salts and bromide generates
silyl radicals and drives the alkylation process.
S. Jung, S. Shin, S. Park, S. Hong, J. Am. Chem. Soc.,
2020, 142, 11370-11375.

A photoinduced intermolecular charge transfer between 1,4-dihydropyridines
and N-amidopyridinium salts induces a single-electron transfer event
without requiring a photocatalyst for the facile C4-functionalization of
pyridines. Alkyl, acyl, and carbamoyl radicals can be generated from
1,4-dihydropyridines, that provide facile access to synthetically valuable
substituted pyridines.
I. Kim, S. Park, S. Hong,
Org. Lett., 2020, 22, 8730-8734.

A Pd-catalyzed decarbonylative Suzuki cross-coupling of widely available
heterocyclic carboxylic acids with arylboronic acids enabled the straightforward
preparation of >45 heterobiaryl products using pyridines, pyrimidines,
pyrazines, and quinolines in very good yields.
A. Cervantes-Reyes, A. C. Smith, G. M. Chinigo, D. C. Blakemore, M. Szostak, Org. Lett.,
2022, 24, 1662-1667.

The use of well-defined and highly reactive [Pd(IPr)(3-CF3-An)Cl2]
(An = aniline) or [Pd(IPr)(cin)Cl] (cin = cinnamyl) Pd(II)-NHC catalysts enables
a a Suzuki-Miyaura cross-coupling of 2-pyridyl ammonium salts to furnish valuable biaryl and heterobiarylpyridines with exceptionally
broad scope that are
ubiquitous in medicinal chemistry and agrochemistry research.
Y. Hu, Y. Gao, J. Ye, Z. Ma, J. Feng, X. Liu, P. Lei, M. Szostak, Org. Lett., 2023, 25,
2975-2980.
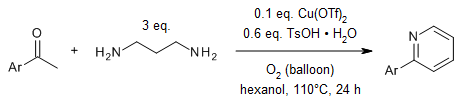
A copper-catalyzed reaction of acetophenones and 1,3-diaminopropane provides
direct access to 2-arylpyridines. A range of electronically diverse
acetophenones undergo this transformation, affording 2-arylpyridines in good
yields.
L.-Y. Xi, R.-Y. Zhang, S. Liang, S.-Y. Chen, X.-Q. Yu, Org. Lett.,
2014,
16, 5269-5271.

Primary amines can be transformed into their corresponding pyridinium salts
in the presence of glutaconaldehyde in acidic medium, including those substrates
that remain unreactive toward the typically used Zincke salt.
G. Asskar, M. Rivard, T. Martens, J. Org. Chem., 2020, 85,
1232-1239.

Two new varieties of solid, moderately air-stable 2-pyridylzinc reagents are
alternatives to unstable or unreliable 2-pyridylboron reagents. Both reagents
can be manipulated in air and are competent nucleophiles in Negishi
cross-coupling reactions.
J. R. Colombe, S. Bernhardt, C. Stathakis, S. L. Buchwald, P. Knochel, Org. Lett., 2013,
15, 5754-5757.

Suzuki reactions of electron-deficient 2-heterocyclic boronates generally give
low conversions and remain challenging. A successful copper(I) facilitated
Suzuki coupling of 2-heterocyclic boronates is broad in scope and affords
greatly enhanced yields of these notoriously difficult couplings. Furthermore,
mechanistic investigations suggest a possible role of copper in the catalytic
cycle.
J. Z. Deng, D. V. Paone, A. T. Ginnetti, H. Kurihara, S. D. Dreher, S. A.
Weissman, S. R. Stauffer, C. S. Burgey, Org. Lett., 2009,
11, 345-347.

A nickel-catalyzed reductive cross-coupling between aryl iodides and
difluoromethyl 2-pyridyl sulfone provides facile access to biaryls under mild
reaction conditions without pregeneration of arylmetal reagents. The new
reactivity of the 2-PySO2CF2H reagent enables C(sp2)-C(sp2)
bond formation through selective C(sp2)-S bond cleavage.
W. Miao, C. Ni, P. Xiao, R. Jia, W. Zhang, J. Hu, Org. Lett., 2021, 23,
711-715.

A Suzuki-Miyaura cross-coupling of tetrabutylammonium 2-pyridyltriolborate salts
with various aryl and heteroaryl chlorides produces the corresponding desired
coupling products with good to excellent yields in the presence of catalytic
amounts of PdCl2dcpp and CuI/MeNHCH2CH2OH in
anhydrous DMF without bases. Tetrabutylammonium 2-pyridyltriolborate salts are
more reactive than the corresponding lithium salts.
S. Sakashita, M. Takizawa, J. Sugai, H. Ito, Y. Yamamoto, Org. Lett., 2013,
15, 4308-4311.

Heteroaromatic tosylates and phosphates are suitable electrophiles in
iron-catalyzed cross-coupling reactions with alkyl Grignard reagents. These
reactions are performed at low temperature allowing good functional group
tolerance with full conversion within minutes.
T. M. Gøgsig, A. T. Lindhardt, T. Skrydstrup, Org. Lett., 2009,
11, 4886-4888.

A simple skeletal editing protocol "inserts" a nitrogen atom into
arylcycloalkenes to form the corresponding N-heterocycles. The use of an
inexpensive cobalt catalyst under aqueous and open-air conditions makes this
protocol very practical. Examples include late-stage modification of compounds
of pharmaceutical interest and complex fused ring compounds.
J. Wang, H. Lu, Y. He, C. Jing, H. Wei, J. Am. Chem. Soc.,
2022, 144, 22433-22439.

A visible-light-enabled biomimetic aza-6π electrocyclization provides diverse
pyridines. In a subsequent Minisci-type reaction, a broad spectrum of
polysubstituted picolinaldehydes were readily constructed with high efficacy and
good functional group tolerance under metal- and oxidant-free conditions under
visible light irradiation.
Q.-L. Zahng, Q.-q. Yu, L. Ma, X. Lu, Q.-T. Fan, T.-S. Duan, Y. Zhou, F.-L.
Zhang, J. Org. Chem., 2021, 86,
17244-17248.

A reaction sequence involving a Wittig reaction, a Staudinger reaction, an
aza-Wittig reaction, a 6π-3-azatriene electrocyclization, and a 1,3-H shift
enables a quick one-pot synthesis of polysubstituted pyridines in very good
yields from aldehydes, phosphorus ylides, and propargyl azide.
H. Wei, Y. Li, K. Xiao, B. Cheng, H. Wang, L. Hu, H. Zhai, Org. Lett.,
2015,
17, 5974-5977.

An efficent cyclization of readily available α,β,γ,δ-unsaturated ketones with
ammonium formate under air atmosphere provides asymmetrical 2,6-diarylpyridines.
The reaction is metal-free and operationally convenient.
Y. Gao, R. Chen, Y. Ma, Synthesis, 2019, 51,
3875-3882.

The combination of iodine and triethylamine triggers an oxime-based synthesis of
2-aryl-substituted pyridines with high chemo-selectivity and wide functional
group tolerance. A broad range of functional pyridines were prepared in good
yields using this metal-free protocol. While neither iodine nor triethylamine
could trigger this transformation, mechanistic experiments indicated a radical
pathway for the reaction.
H. Huang, J. Cai, L. Tang, Z. Wang, F. Li, G.-J. Deng, J. Org. Chem.,
2016,
81, 1499-1505.

A redox-neutral, [3+3]-type condensation of O-acetyl ketoximes and
α,β-unsaturated aldehydes, that is synergistically catalyzed by a copper(I) salt
and a secondary ammonium salt (or amine), allows modular synthesis of a variety
of substituted pyridines under mild conditions with tolerance of a broad range
of functional groups. The reaction is driven by a merger of iminium catalysis
and redox activity of the copper catalyst.
Y. Wei, N. Yoshikai, J. Am. Chem. Soc., 2013,
135, 3756-3759.

Cationic half-sandwich rare-earth catalysts provide an efficient, general
and atom-economical method for the synthesis of 2-alkylated pyridine derivatives
via C-H addition to olefins. A wide range of pyridine and olefin substrates
including α-olefins, styrenes, and conjugated dienes are compatible with the
catalysts.
B.-T. Guan, Z. Hou, J. Am. Chem. Soc., 2011,
133, 18066-18089.

The use of Pd2(dba)3 and X-Phos as a ligand enables a mild
Negishi cross-coupling of 2-heterocyclic organozinc reagents and aryl chlorides
providing 2-aryl-substituted pyridines and thiophenes in high yields. An
efficient method to generate the organozinc reagents at room temperature is also
demonstrated.
M. R. Luzung, J. S. Patel, J. Yin, J. Org. Chem., 2010,
75, 8330-8332.

An efficient lithiation/isomerization/intramolecular carbolithiation sequence
provides a divergent and straightforward entry to a wide range of
polysubstituted dihydropyridines and pyridines starting from readily available
N-allyl-ynamides.
W. Gati, M. M. Rammah, M. B. Rammah, F. Couty, G. Evano, J. Am. Chem. Soc., 2012,
134, 9078-9081.

The olefin cross-metathesis reaction provides a rapid and efficient method for
the synthesis of α,β-unsaturated 1,5-dicarbonyl derivatives which then serve as
effective precursors to pyridines with a wide range of substitution patterns.
High levels of regiocontrol, short reaction sequences, and facile substituent
variation are all notable aspects of this methodology.
T. J. Donohoe, J. A. Basutto, J. F. Bower, A. Rathi, Org. Lett., 2011,
13, 1036-1039.

Regioselective hydroamination of alkynes with N-silylamine using a bis(amidate)bis(amido)titanium(IV) precatalyst,
addition of α,β-unsaturated
carbonyls to the crude mixture followed by oxidation affords 47 examples of
pyridines in good yields containing variable substitution patterns, including
pharmaceutically relevant 2,4,5-trisubstituted pyridines.
E. K. J. Lui, D. Hergesell, L. L. Schafer, Org. Lett.,
2018, 20, 6663-6667.

A very sterically hindered N-heterocyclic carbene ligand promotes
cross-coupling at C4 of 2,4-dichloropyridines with high selectivity (∼10:1).
Under optimized conditions, diverse substituted 2,4-dichloropyridines and
related compounds undergo cross-coupling to form C4-C(sp2) and C4-C(sp3) bonds
using organoboron, -zinc, and -magnesium reagents.
J. P. Norman, N. G. Larson, E. D. Entz, S. R. Neufeldt, J. Org. Chem., 2022, 87,
7414-7421.

A photoredox coupling of α,α-difluoro-β-iodoketones with silyl enol ethers
catalyzed by fac-Ir(ppy)3 under blue LED irradiation with subsequent
one-pot condensation with ammonium acetate provides diversely substituted
3-fluoropyridines.
S. I. Scherbinina, O. V. Fedorov, V. V. Levin, V. A. Kokorekin, M. I. Struchkova,
A. D. Dilman, J. Org. Chem.,
2017, 82, 12967-12974.

A convenient base-promoted reaction of 1-arylethylamines with ynones gives
polysubstituted pyridines via direct β-C(sp3)-H functionalization of
enaminones under metal-free conditions. This procedure features high
regioselectivity, high efficiency, and environmental friendliness. Various
polysubstituted pyridines were provided in high yields.
J. Shen, D. Cai, C. Kuai, Y. Liu, M. Wei, G. Cheng, X. Cui, J. Org. Chem.,
2015,
80, 6584-6589.

Ring-closing olefin metathesis (RCM)/elimination and RCM/oxidation/deprotection
of nitrogen-containing dienes are the key processes of new synthetic routes to
substituted 3-hydroxypyridines. An application of RCM/oxidation/deprotection
allows the synthesis of 3-aminopyridine derivatives.
K. Yoshida, F. Kawagoe, K. Hayashi, S. Horiuchi, T. Imamoto, A. Yanagisawa, Org. Lett., 2009,
11, 515-518.

A visible-light-enabled biomimetic aza-6π electrocyclization provides diverse
pyridines. In a subsequent Minisci-type reaction, a broad spectrum of
polysubstituted picolinaldehydes were readily constructed with high efficacy and
good functional group tolerance under metal- and oxidant-free conditions under
visible light irradiation.
Q.-L. Zahng, Q.-q. Yu, L. Ma, X. Lu, Q.-T. Fan, T.-S. Duan, Y. Zhou, F.-L.
Zhang, J. Org. Chem., 2021, 86,
17244-17248.

A simple and highly efficient protodecarboxylation of various heteroaromatic
carboxylic acids is catalyzed by Ag2CO3 and AcOH in DMSO.
This methodology enables also a selective monoprotodecarboxylation of several
aromatic dicarboxylic acids.
P. Lu, C. Sanchez, J. Cornella, I. Larrosa, Org. Lett., 2009,
11, 5710-5713.

Reactions of vinyl azides with monocyclic cyclopropanols provided pyridines in
the presence of Mn(acac)3, whereas those with bicyclic cyclopropanols
led to the formation of 2-azabicyclo[3.3.1]non-2-en-1-ol derivatives using a
catalytic amount of Mn(acac)3.
Y.-F. Wang, S. Chiba, J. Am. Chem. Soc., 2009,
131, 12570-12572.

A ruthenium-catalyzed formal dehydrative [4 + 2] cycloaddition of enamides and
alkynes enables a mild and economic construction of a broad range of highly
substituted pyridines. The reaction tolerates many functional groups and offers
excellent regioselectivities.
J. Wu, W. Xu, Z.-X. Yu, J. Wang, J. Am. Chem. Soc., 2015,
137, 9489-9495.

A DBU-promoted metal-free reaction of 2-allyl-2H-azirines affords
1-azatrienes that in situ electrocyclize to pyridines in very good yields. The
reaction displays a broad substrate scope and tolerates various substituents. In
addition, one-pot synthesis of pyridines from oximes via in situ formation of 2H-azirines
was achieved.
Y. Jiang, C.-M. Park, T.-P. Loh, Org. Lett., 2014,
16, 3432-3435.

An iodoxybenzoic acid-mediated selected oxidative cyclization of N-hydroxyalkyl
enamines provides a variety of 2,3-disubstituted pyrroles and pyridines in good
selectivity. This metal-free method offers use of environmentally friendly
reagents, broad substrate scope, mild reaction conditions, and high efficiency.
P. Gao, H.-J. Chen, Z.-J. Bai, M.-N. Zhao, D. Yang, J. Wang, N. Wang, L. Du,
Z.-H. Guan, J. Org. Chem., 2020, 85,
7939-7951.

An efficient and practical visible-light photoredox-catalyzed formal [5 + 1]
cycloaddition of N-tosyl vinylaziridines with difluoroalkyl halides as
unique C1 synthons provides pyridines in good yields.
Y. Liu, W. Luo, Z. Wang, Y. Zhao, J. Zhao, X. Xu, C. Wang, P. Li, Org. Lett., 2020, 22,
9638-9643.

Oxidative one-pot sequential reactions of inactivated saturated ketones with
electron-deficient enamines enable an efficient synthesis of 3-acylpyridines and
pyridine-3-carboxylates. The reaction involve oxidative dehydrogenation of the
saturated ketone substrate, followed by [3+3] annulation with β-enaminone or
β-enaminoester via a cascade process, including Michael addition, aldol type
condensation, and oxidative aromatization.
G. Chen, Z. Wang, X. Zhang, X. Fan, J. Org. Chem.,
2017, 82, 11230-11237.

A 2-fluoro-1,3-dicarbonyl-initiated one-pot Michael addition/[5 + 1]
annulation/dehydrofluorinative aromatization reaction sequence enables a
transition-metal catalyst-free, regioselective synthesis of di-, tri-, tetra-,
and pentasubstituted pyridines as well as fused pyridines from readily available
starting materials.
Z. Song, X. Huang, W. Yi, W. Zhang, Org. Lett.,
2016, 18, 5640-5643.

A one-pot synthesis of substituted pyridines via a domino cyclization-oxidative
aromatization approach is based on the use of a new bifunctional noble
metal-solid acid catalyst, Pd/C/K-10 montmorillonite and microwave irradiation.
The cyclization readily takes place on the strong solid acid while palladium
dehydrogenates the dihydropyridine intermediate.
O. De Paolis, J. Baffoe, S. M. Landge, B. Török, Synthesis, 2008,
3423-3428.

Stable 1,2,3-triazine 1-oxides are remarkably effective substrates for
inverse electron demand Diels-Alder reactions. Base-catalyzed reactions with
amidines provide pyrimidines, with β-ketocarbonyl compounds and related nitrile
derivatives polysubstituted pyridines and with 3/5-aminopyrazoles pyrazolo[1,5-a]pyrimidines
in high yield at room temperature.
S. Biswas, L. De Angelis, G. Rivera, H. Arman, M. P. Doyle, Org. Lett., 2023, 25,
1104-1108.

An efficient copper-mediated cleavage of isoxazoles enables the synthesis of
nicotinate derivatives and tetrasubstituted pyridines in DMSO as solvent. DMSO
serves as a one-carbon surrogate, that forms two C-C bonds.
P. Kumar, M. Kapur,
Org. Lett., 2020, 22, 5855-5860.

A simple, modular method to prepare highly substituted pyridines in good
isolated yields employs a cascade reaction comprising a novel Cu-catalyzed
cross-coupling of alkenylboronic acids with α,β-unsaturated ketoxime O-pentafluorobenzoates,
electrocyclization of the resulting 3-azatriene, and air oxidation.
S. Liu, L. S. Liebeskind, J. Am. Chem. Soc., 2008,
130, 6918-6919.

A single-step conversion of various N-vinyl and N-aryl amides
to the corresponding pyridine and quinoline derivatives involves amide activation with trifluoromethanesulfonic anhydride in the
presence of 2-chloropyridine followed by π-nucleophile addition to the activated
intermediate and annulation. Compatibility of this chemistry with various functional groups is
noteworthy.
M. Movassaghi, M. D. Hill, O. K. Ahmad, J. Am. Chem. Soc.,
2007,
129, 10096-10097.

DABCO promotes an efficient, solvent-free, and eco-friendly domino reaction of
various β,γ-unsaturated α-ketocarbonyls with 5/6-membered cyclic sulfamidate
imines in neat conditions under MW irradiation to provide densely functionalized
picolinates in short reaction times.
S. Biswas, D. Majee, S. Guin, S. Samanta, J. Org. Chem.,
2017, 82, 10928-10938.

A domino reaction of 5-membered cyclic sulfamidate imines with various
Morita-Baylis-Hillman acetates of nitroolefins/nitrodienes provides a series of
4,6-diarylpicolinates in excellent yields in the presence of DABCO as an organic
base at 55 °C.
D. Majee, S. Biswas, S. M. Mobin, S. Samanta, J. Org. Chem.,
2016,
81, 4378-4385.

A range of highly functionalised pyridines is prepared from enamino and
alkynones in a single synthetic step by the use of acetic acid or amberlyst 15
ion exchange resin at 50°C.
M. C. Bagley, J. W. Dale, J. Bower, Synlett, 2001,
1149-1151.

N-Propargylic β-enaminones are common intermediates for the
synthesis of polysubstituted pyrroles and pyridines. In the presence of Cs2CO3
N-propargylic
β-enaminones are cyclized to pyrroles in good to high yields, whereas CuBr leads to pyridines.
S. Cacchi, G. Fabrizi, E. Filisti, Org. Lett., 2008,
10, 2629-2632.

Polysubstituted pyridines are prepared in good yield and with total regiocontrol
by the one-pot reaction of an alkynone, 1,3-dicarbonyl compound and ammonium
acetate in alcoholic solvents. This new three-component heteroannulation
reaction proceeds under mild conditions in the absence of an additional acid
catalyst.
X. Xiong, M. C. Bagley, K. Chapaneri, Tetrahedron Lett., 2004,
45, 6121-6124.

Tri- or tetrasubstituted pyridines are prepared by microwave irradiation of
ethyl β-aminocrotonate and various alkynones in a single synthetic step and with
total control of regiochemistry. This new one-pot Bohlmann-Rahtz procedure
conducted at 170°C gives superior yields to similar experiments conducted using
conductive-heating techniques in a sealed tube.
M. C. Bagley, R. Lunn, X. Xiong, Tetrahedron Lett., 2002,
43, 8331-8334.

The direct conversion of amides, including sensitive N-vinyl amides,
to the corresponding trimethylsilyl alkynyl imines followed by a
ruthenium-catalyzed protodesilylation and cycloisomerization gives various
substituted pyridines and quinolines.
M. Movassaghi, M. D. Hill, J. Am. Chem. Soc.,
2006, 128, 4592-4593.

A rhodium-catalyzed chelation-assisted C-H activation of α,β-unsaturated
ketoximes and the reaction with alkynes affords highly substituted pyridine
derivatives.
K. Parthasararathy, M. Jeganmohan, C.-H. Cheng, Org. Lett., 2008,
10, 325-328.

A convenient one-pot C-H alkenylation/electrocyclization/aromatization sequence
allows the synthesis of highly substituted pyridine derivatives from alkynes and
α,β-unsaturated N-benzyl aldimines and ketimines. The reaction proceeds
through dihydropyridine intermediates.
D. A. Colby, R. G. Berman, J. A. Ellman, J. Am. Chem. Soc., 2008,
130, 3645-3651.

The NH4I-triggered formal [4 + 2] annulation of α,β-unsaturated
ketoxime acetates with N-acetyl enamides enables an efficient and
straightforward construction of polysubstituted pyridines in good yields. This
metal-free protocol employs electron-rich enamides as C2 synthons and tolerates
a wide range of functional groups.
J. Duan, L. Zhang, G. Xu, H. Chen, X. Ding, Y. Mao, B. Rong, N. Zhu, K. Guo, J. Org. Chem., 2020, 85,
8157-8165.

A concise copper-catalyzed N-O bond cleavage/C-C/C-N bond formation procedure
enables the synthesis of multisubstituted pyridines from various oxime acetates,
activated methylene compounds, and a wide range of aldehydes. This method
features inexpensive catalysts, no need for extra oxidant, and high step-economy.
H. Jiang, J. Yang, X. Tang, J. Li, W. Wu, J. Org. Chem.,
2015,
80, 8763-8771.

A concise one-pot synthesis of highly functionalized pyridines involves a formal
insertion of rhodium vinylcarbenoids derived from diazo compounds across the N-O
bond of isoxazoles. Upon heating, the insertion products undergo a rearrangement
to give 1,4-dihydropyridines. DDQ oxidation then affords the corresponding
pyridines in good yield.
J. R. Manning, H. M. L. Davies, J. Am. Chem. Soc., 2008,
130, 8602-8603.

Cationic rhodium(I)/modified-BINAP complexes catalyze a chemo- and
regioselective [2+2+2] cycloaddition of a wide variety of alkynes and
nitriles leading to highly functionalized pyridines under mild reaction
conditions.
K. Tanaka, N. Suzuki, G. Nishida, Eur. J. Org. Chem., 2006,
3917-3922.

Conversion of unsaturated ketones and aldehydes derived from the
cycloisomerization of primary and secondary propargyl diynols in the presence of
[CpRu(CH3CN)3]PF6 to 1-azatrienes and a
subsequent electrocyclization-dehydration provides pyridines with excellent
regiocontrol.
B. M. Trost, A. C. Gutierrez, Org. Lett., 2007,
9, 1473-1476.

Coupling of acetylene, nitrile, and a titanium reagent generated new
azatitanacyclopentadienes in a highly regioselective manner. The subsequent
reaction with sulfonylacetylene and electrophiles gave substituted pyridines
virtually as a single isomer. Alternatively, the reaction of
azatitanacyclopentadienes with an aldehyde or another nitrile gave furans or
pyrroles having four different substituents again in a regioselective manner.
D. Suzuki, Y. Nobe, R. Tanaka, Y. Takayama, F. Sato, H. Urabe, J. Am. Chem. Soc.,
2005, 127, 7474-7479.

A mild, efficient, and general aromatization of Hantzsch 1,4-dihydropyridines
with oxygen was realized at room temperature with 5 mol % of
9-phenyl-10-methylacridinium perchlorate as photocatalyst, which could be easily
recovered and reused.
X. Fang, Y.-C. Liu, C. Li, J. Org. Chem., 2007,
72, 8608-8610.

In the presence of activated carbon, Hantzsch 1,4-dihydropyridines and
1,3,5-trisubstituted pyrazolines were aromatized with molecular oxygen to the
corresponding pyridines and pyrazoles in excellent yields.
N. Nakamichi, Y. Kawashita, M. Hayashi, Synthesis, 2004,
1015-1020.

4-Substituted-1,4-dihydropyridines are readily and efficiently aromatized in
only one minute using commercial manganese dioxide in the absence of an
inorganic support at 100 °C under microwave irradiation. This rapid
procedure gives the dehydrogenated or 4-dealkylated product in excellent
yield.
M. C. Bagley, M. C. Lubinu, Synthesis, 2006, 1283-1288.

Hantzsch 1,4-dihydropyridines undergo smooth aromatization catalyzed by
iodoxybenzoic acid (IBX) to afford the corresponding pyridine derivatives in
high yields. All the reactions were carried out in DMSO solvent at 80-85 °C
for a period of two to four hours to complete conversion of the substrates.
J. S. Yadav, B. V. S. Reddy, A. K. Basak, G. Baishya, A. V. Narsaiah, Synthesis, 2006, 451-454.

An intermolecular, Rh(III)-catalyzed cyclization of oximes and diazo
compounds involving tandem C-H activation, cyclization, and condensation steps
gives multisubstituted isoquinoline and pyridine N-oxides under mild
conditions. The reaction obviates the need for oxidants, releases N2
and H2O as the byproducts, and displays a broad substituent scope.
Z. Shi, D. C. Koester, M. Boultadakis-Arapinis, F. Glorius, J. Am. Chem. Soc., 2013,
135, 12204-12205.

Trapping of in situ generated active intermediate 1,4-oxazepines, formed from
base-promoted 7-exo-dig cyclization reaction of N-propargyl
enaminones, with alcohols/thiols and aldehydes provides
2-alkoxy/2-sulfenylpyridines and dihydrofuro[2,3-b]pyridines in good
yields within 30 min at room temperature. This cascade reaction generates 1
equiv of H2O as the sole byproduct.
G. Cheng, L. Xue, Y. Weng, X. Cui, J. Org. Chem.,
2017, 82, 9515-9524.

A K2CO3-mediated cyclization and rearrangement of
γ,δ-alkynyl oximes for the synthesis of pyridols employs readily accessible
starting materials, tolerates a wide range of functional groups, and gives
various synthetically challenging pyridols in good yields. The reaction proceeds
via an efficient [1,3] rearrangement of an O-vinyl oxime intermediate
which is in situ generated by intramolecular nucleophilic addition of
γ,δ-alkynyl oximes.
S. Wang, Y.-Q. Guo, Z.-H. Ren, Y.-Y. Wang, Z.-H. Guan, Org. Lett.,
2017, 19, 1574-1577.

Pyridine N-oxides were converted to 2-aminopyridines in a one-pot fashion
using Ts2O-tBuNH2 followed by in situ deprotection
with TFA. The amination proceeded in high yields, excellent 2-/4-selectivity,
and with good functional group compatibility.
J. Yin, B. Xiang, M. H. Huffman, C. E. Raab, I. W. Davies, J. Org. Chem., 2007,
72, 4554-4557.

A multifunctional reagent enables a direct conversion of pyridines to
Boc-protected 2-aminopyridines with exquisite site selectivity and
chemoselectivity under mild conditions without precautions toward air or
moisture. The reaction tolerates nearly all common functionality.
P. S. Fier, S. Kim, R. D. Cohen, J. Am. Chem. Soc.,
2020, 142, 8614-8618.

In a ligand-free chromium(II)-catalyzed amination reaction of various N-heterocyclic
chlorides, CrCl2 regioselectively catalyzes the reaction of
chloropyridines, chloroquinolines, chloroisoquinolines, and chloroquinoxalines
with a broad range of magnesium amides in the presence of lithium chloride as
additive. The reactionse provide the desired aminated products in good yield.
A. K. Steib, S. Fernandez, O. M. Kuzmina, M. Corpet, C. Gosmini, P. Knochel,
Synlett, 2015, 26, 1049-1054.

Base-mediated cascade reactions of α,β-unsaturated ketones and 1,1-enediamines,
which include Michael addition, intramolecular cyclization, aromatization, and a
base-dependent optional loss of HNO2, provide
2-amino-4,6-diarylpyridine derivatives. The methods are suitable for efficient
parallel synthesis of pyridines.
Q. Luo, R. Huang, Q. Xiao, Y. Yao, J. Lin, S.-J. Yan, J. Org. Chem., 2019, 84,
1999-2011.

The use of the commercially available N-fluorobenzenesulfonimide (NFSI)
as an amination reagent enables a copper-catalyzed aminative aza-annulation of
enynyl azide to provide amino-substituted nicotinate derivatives in a single
step in good yield.
C. R. Reddy, S. K. Prajapti, R. Ranjan, Org. Lett.,
2018, 20, 3128-3131.

Condensation of
2,4-dioxo-carboxylic acid ethyl esters with ethyl 3-amino-3-iminopropionate
hydrochloride provides a wide variety of mono- or
disubstituted 2-amino isonicotinic acids. The reaction likely proceeds through an in situ decarboxylation
process.
X. Jin, L. Xing, D. D. Deng, Y. Yan, Y. Fu, W. Dong, J. Org. Chem., 2022, 87,
1541-1544.

An efficient protecting-group-free two-step route to a broad range of aza- and
diazaindoles was established, starting from chloroamino-N-heterocycles.
The method involves an optimized Suzuki-Miyaura coupling with
(2-ethoxyvinyl)borolane followed by acetic acid-catalyzed cyclization.
D. K. Whelligan, D. W. Thomson, D. Taylor, S. Hoelder, J. Org. Chem., 2010,
75, 11-15.

Nine azidopyridines bearing a single fluorine, chlorine, or bromine atom were
prepared and examined by differential scanning calorimetry (DSC). The utility of
these versatile intermediates was demonstrated through their use in a variety of
Click reactions and the diversification of the halogen handles.
M. D. Mandler, A. P. Degnan, S. Zhang, D. Aulakh, K. Georges, B. Sandhu, A.
Sarjeant, Y. Zhu, S. C. Traeger, P. T. Cheng, B. A. Ellsworth, A. Regueiro-Ren, Org. Lett., 2022,
24,
799-803.

A Gold(I)-catalyzed hetero-tetradehydro-Diels-Alder cycloaddition of
enynamides and cyanamides provides diversely substituted 2,6-diaminopyridines in
very good yields. This efficient reaction proceeds under very mild conditions with high functional group tolerance.
N. V. Shcherbakov, D. V. Dar'in, Y. Y. Kukushkin, Y. Y. Dubovtsev, J. Org. Chem., 2021, 86,
7218-7228.

[bmim]OH, a basic ionic liquid, efficiently promotes a one-pot condensation of
aldehydes, malononitrile, and thiophenols to produce highly substituted
pyridines in high yields. The ionic liquid can be recovered and recycled.
B. C. Ranu, R. Jana, S. Sowmiah, J. Org. Chem., 2007,
72, 3152-3154.