Categories: Synthesis of S-Heterocycles, C-S Bond Formation > Synthesis of Cyclic Sulfides >
Synthesis of thiiranes
Recent Literature
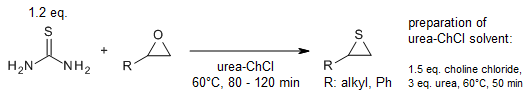
A general, straightforward and odourless ring-opening reaction allows the
preparation of β-hydroxy sulfides from in situ generated S-alkylisothiouronium
salts in urea-choline chloride-based deep eutectic solvent (DES). The reaction
of epoxides with thiourea in DES yields the corresponding thiiranes.
N. Azizi, Z. Yadollahy, A. Rahimzadeh-oskooee,
Synlett, 2014, 25, 1085-1088.

Thiiranes are obtained in excellent yields and with high
diastereoselectivity upon microwave irradiation of a mixture of
α-haloketones, O,O-diethyl hydrogen phosphorodithioate and
alumina-supported sodium borohydride in solvent-free conditions.
L. D. S. Yadav, R. Kapoor, Synthesis, 2002, 2344-2346.